How do atmospheric pressure and elevation affect boiling point?
1 Answer
- As elevation increases, atmospheric pressure and boiling point decrease.
Explanation:
-
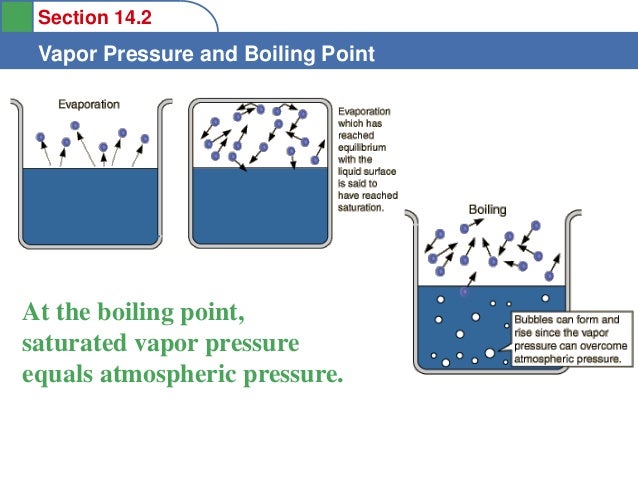
Boiling point is the point at which vapour pressure equals atmospheric pressure.
-
In a liquid, some particles always have enough energy to escape to the gas phase. Gaseous particles are also returning to the liquid.
-
The vapour pressure is the pressure exerted by the gas when the amount of particles leaving the liquid equals the amount of particles entering the liquid
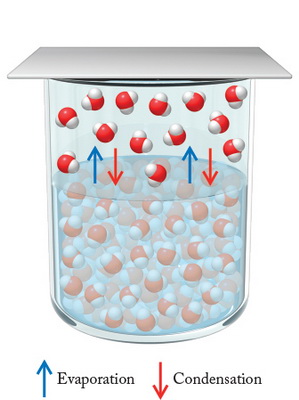
(from facweb.bhc.edu)
- As temperature increases, more particles have enough energy to escape to the gas phase. This increases the vapour pressure. When the vapour pressure equals atmospheric pressure, the liquid boils.
As elevation increases, atmospheric pressure decreases because air is less dense at higher altitudes.
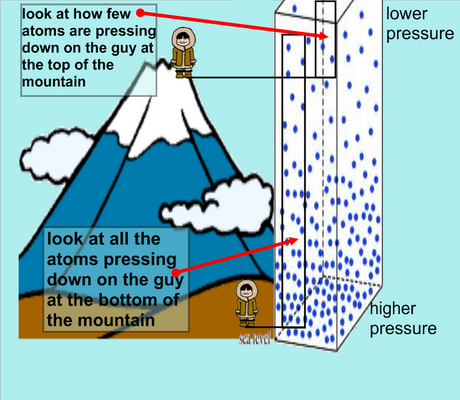
Because the atmospheric pressure is lower, the vapour pressure of the liquid needs to be lower to reach boiling point.
Therefore, less heat is required to make the vapour pressure equal to the atmospheric pressure. The boiling point is lower at higher altitude.
Here's a video that demonstrates the effect of atmospheric pressure on boiling point.

