What causes gas pressure (in terms of kinetic theory)?
1 Answer
Gas pressure is caused by the collisions of gas particles with the walls of the container.
Explanation:
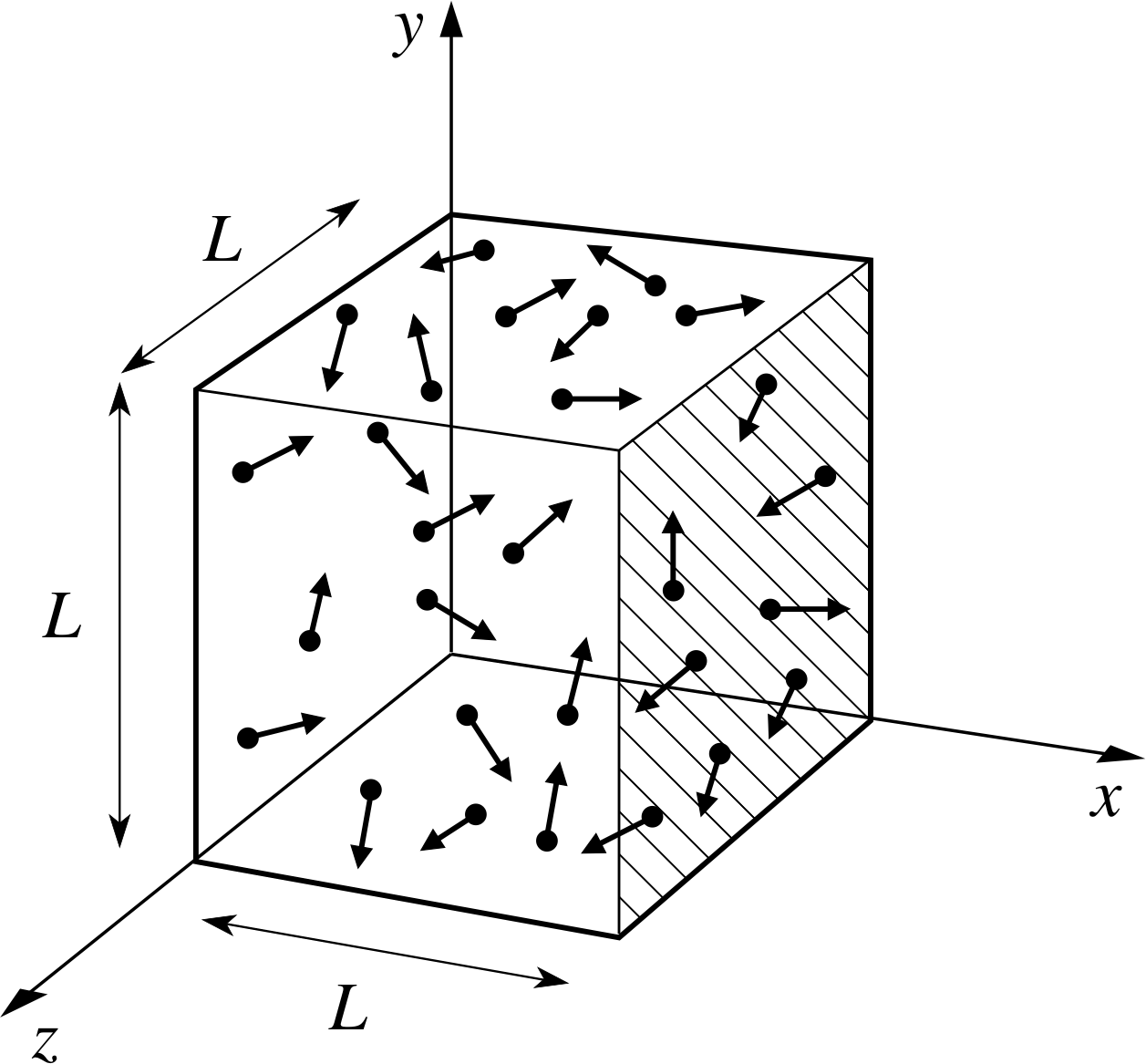
According to kinetic theory, molecules inside a volume (e.g. a balloon) are constantly moving around freely.
During this molecular motion, they constantly collide with each other and with the walls of the container.
In a small balloon, that would be many thousands of billions of collisions each second.
The force of impact of a single collision is too small to measure.
However, taken all together, this large number of impacts exerts a considerable force on the surface of the container.
If they hit the surface of the balloon straight on (at a 90 ° angle), they exert their maximum force.
If they hit the surface at an angle less than 90 °, they exert a smaller force.
The sum of all these forces causes the pressure,

The diagram above represents a balloon containing molecules of a gas (the red dots).
The yellow arrows indicate that the gas pressure,
The larger the number of collisions per area of the container, the larger the pressure:
Pressure =
The direction of this force is always perpendicular to the surface of the container at every point.
The video below gives a good explanation of gas pressure.

