What is an example of a supersaturated solution practice problem?
1 Answer
Usually the instructor gives you a table of solubility curves. You must then state if a solution is unsaturated, saturated, or supersaturated.
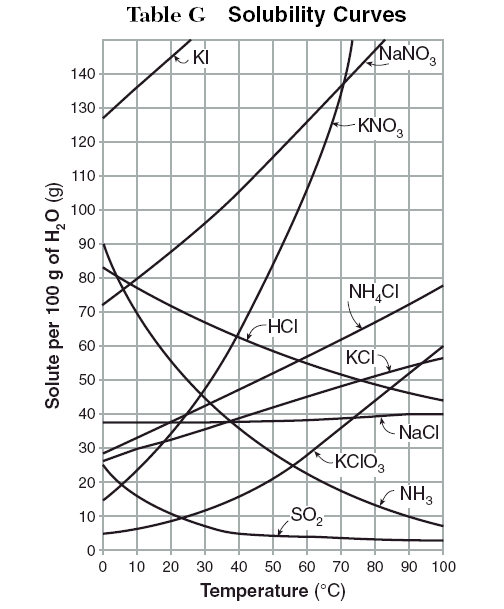
Examples
The questions below give you an amount of solute is given and a temperature. If all the solute could be dissolved in 100 g of water, would the resulting solution be unsaturated, saturated, or supersaturated?
1. 60 g KCl at 60 °C.
2. 10 g KClO₃ at 60 °C.
3. 80 g NaNO₃ at 10 °C.
4. 80 g HCl at 20 °C.
5. 55 g NH₃ at 20 °C.
6. 60 g KClO₃ at 60 °C.
7. 125 g KNO₃ at 60 °C.
8. 65 g NH₄Cl at 100 °C.
9. 35 g NaCl at 100 °C
1. Supersaturated; saturation = 45 g KCl
2. Unsaturated; saturation = 28 g KClO₃
3. Saturated; saturation = 10 g NaNO₃
4. Supersaturated; saturation = 72 g HCl
5. Saturated; saturation = 55 g NH₃
6. Supersaturated; saturation = 28 g KClO₃
7. Supersaturated; saturation = 107 g KNO₃
8. Unsaturated; saturation = 78 g NH₄Cl
9. Unsaturated; saturation = 40 g NaCl

