Question #2f835
1 Answer
Butanoic acid is not an alcohol. The -OH group is part of the -COOH group which makes it a carboxylic acid.
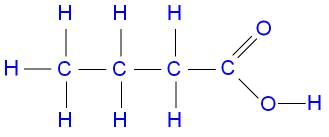
To work out what class of alcohol an alcohol belongs to, find the carbon atom that the -OH group is connected to. Now look at all the other bonds from that carbon atom and count the number of these bonds that go to other carbon atoms. That number is the class of the alcohol.
For example, butan-1-ol (1-butanol) is a primary alcohol because it's structure is
Butan-2-ol (2-butanol) is a secondary alcohol because it's structure is
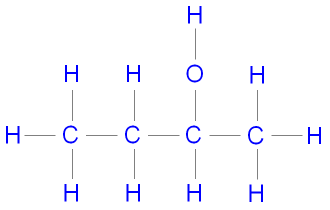
It cannot be made by reducing a carboxylic acid (only primary alcohols can) but can be made by reducing butanone, which is a ketone. Dilute sodium borohydride would be a suitable reducing agent.


