What are the limitations of the octet rule?
1 Answer
The octet rule states that atoms can combine either by transferring or by sharing their valence electrons to attain an octet of electrons in their valence shell.
Explanation:
One limitation of the octet rule is that it cannot be applied to the nonmetals after silicon in the Periodic Table.
These elements can “expand their octet” and have more than eight valence electrons around the central atom.
Examples are
The
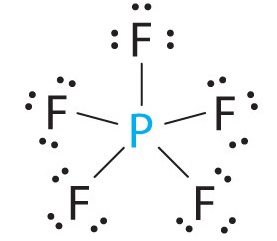
The
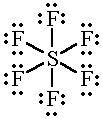
And so does the

Molecules with an odd number of electrons such as
One atom must have an odd number of electrons.
The

(from file.scirp.org)
In
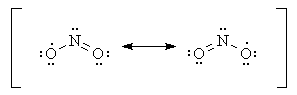
In some molecules the central atom cannot possibly have eight valence electrons. For example,

And



