How can I write the formula for potassium nitride?
1 Answer
The correct answer is
To see how we arrived at that answer, we first have to examine the electronic configuration of
As potassium obeys the octet rule, it will lose one electron in its
Electronic configuration of
Nitrogen also obeys the octet rule and will gain three electrons to achieve stable octet noble gas configuration, forming the negative nitride ion,
Electronic configuration of
Each nitrogen atom gains three electrons from three potassium atoms and each potassium atom loses one electron to a nitrogen atom. In this process each potassium atom becomes
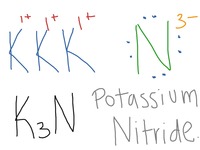
The first 1:30 of this video shows the movement of the electrons in potassium nitride (wordlessly).


