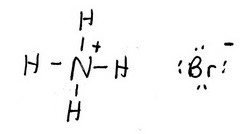Question #04cc4
1 Answer
Here are the steps that I follow when drawing a Lewis structure.
- Decide which atom is the central atom in the structure.
- Draw a skeleton structure in which the other atoms are single-bonded to the central atom.
- Draw a trial structure by putting electron pairs around every atom until each gets an octet.
- Count the valence electrons in your trial structure.
- Now, count the valence electrons you actually have available.
- Add or remove electrons in the trial structure to match the available electrons.
- Assign formal charges to each atom.
NH₄Br is an ionic compound. It consists of NH₄⁺ ions and Br⁻ ions. We must draw separate Lewis structures for these ions.
Lewis Structure of NH₄⁺
The trial structure is
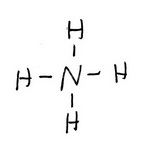
The trial structure contains 8 electrons.
Electrons available = 5 + 4(1) - 1 = 8. The trial structure is the correct structure.
Formal charges: N = 5 - ½(8) = +1; H = 1 - ½(2) = 0.
The Lewis structure of NH₄⁺ is
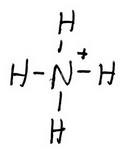
Lewis Structure of Br⁻
The trial structure is
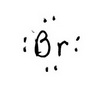
The trial structure contains 8 electrons.
Electrons available = 7 + 1 = 8.
The trial structure is the correct structure. The formal charge is -1.
The Lewis structure of Br⁻ is
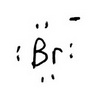
Lewis Structure of NH₄Br
We write the two structures side by side. The Lewis structure is