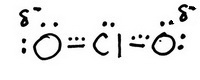How can I draw the Lewis structure for ClO2-?
1 Answer
Here's how I would do it.
Explanation:
You can find the procedure here.
For
The skeleton structure is
The trial structure is
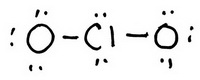
You have 20 valence electrons in your trial structure.
The valence electrons you have available are:
Hence, the trial structure has the correct number of electrons.
The formal charge on each atom is:
Every atom has a formal charge.
We can reduce the number of formal charges by moving a lone pair of electrons from
This gives us two new structures in which one
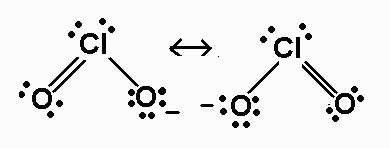
The actual structure is nine of these. Rather, it is a resonance hybrid of them both