How do isotopes become stable?
1 Answer
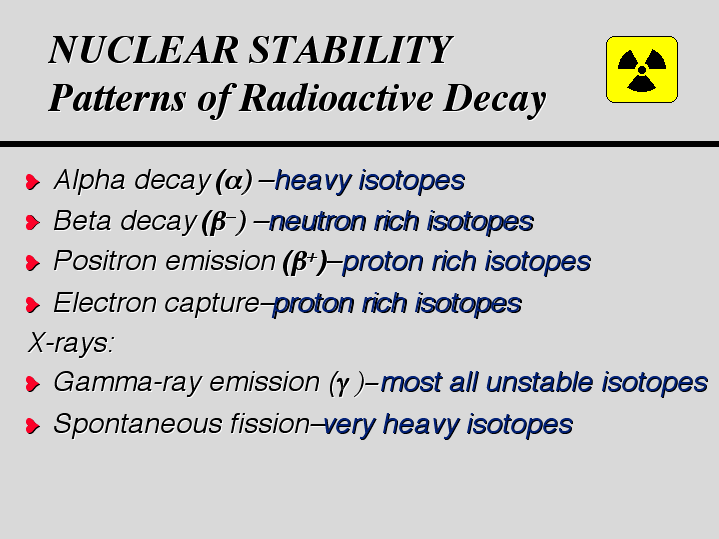
Most isotopes become stable by emitting alpha particles, beta particles, positrons, or gamma rays. A few become stable by electron capture or by spontaneous fission.
ALPHA PARTICLES
An alpha particle is a helium nucleus. The heavier elements usually decay by alpha emission. For example,
BETA PARTICLES
A beta particle is an electron. Nuclei that have too few protons can decay by converting a neutron to a proton and emitting a beta particle. For example,
₁₅³²P → ₁₆³²S + ₋₁⁰e
POSITRONS
A positron is the antimatter counterpart of an electron. Nuclei that have too many protons can decay by converting a proton to a neutron and emitting a positron. For example,
GAMMA RAYS:
Gamma rays are high-energy photons. When a nucleus emits an alpha or beta particle, the new nucleus may have excess energy. It can release this excess energy by emitting gamma rays.
Thus, thorium-234 becomes more stable by releasing gamma rays and a beta particle.
₉₀²³⁴Th → ₉₁²³⁴Pa + ₋₁⁰e + γ
ELECTRON CAPTURE
An isotope with too many protons in the nucleus may capture an electron. A proton becomes a neutron, so the atomic number decreases but the atomic mass stays the same.
An example of electron capture is the decay of beryllium-7.
₄⁷Be + ₋₁⁰e → ₃⁷Li
SPONTANEOUS FISSION
Nuclei with mass numbers greater than 230 often become stable by spontaneous fission.
For example