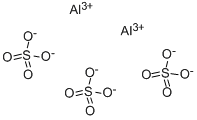
Question #2a028
1 Answer
Aluminum sulfate is an ionic compound, so the procedure is like that in
http://socratic.org/questions/what-is-the-lewis-structure-of-nh4br
The formula of aluminum sulfate is Al₂(SO₄)₃. It consists of Al³⁺ ions and SO₄²⁻ ions.
The Lewis structure for the aluminum ion is Al³⁺.
The trial structure for sulfate ion is
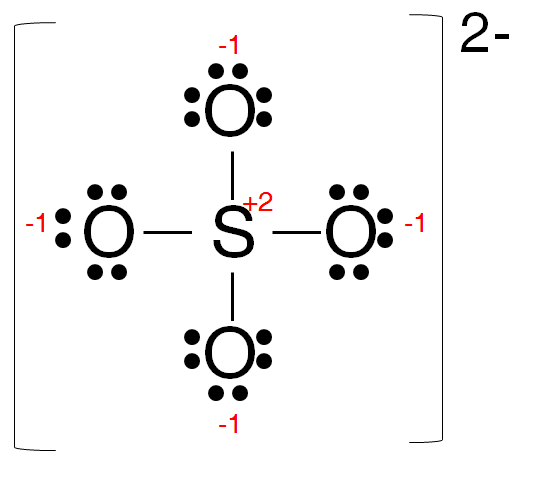
It has 32 electrons.
The number of electrons available is 6 + 4×6 + 2 = 32. So the trial structure has the correct number of electrons
Every atom in the structure has a formal charge. We can reduce the number of formal charges by inserting double bonds, as in
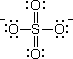
There are six possible structures, differing only in the positions of the double bonds. The sulfate ion is a resonance hybrid of these structures.
For convenience, we will use only one of the contributing structures in our formulas.
Since we need two Al³⁺ ions and three SO₄²⁻ ions, the Lewis structure of aluminum sulfate is