What is an example of a practice problem with isotopes?
1 Answer
Isotopes are atoms of the same element that have different mass numbers. The mass number of an isotope is the sum of the protons and neutrons in its nucleus. Each element has its own unique atomic number, which is the number of protons in its atomic nuclei, so the number of protons in all of the isotopes of a given element is the same. However, the number of neutrons can vary, and that is the reason for the different mass numbers for different isotopes of the same element.
There are specific symbols that represent the mass number, atomic number, and number of neutrons: X represents the symbol of the element, Z represents the atomic number (number of protons), A represents the mass number, and N represents the neutrons.
There are several equations related to isotopes:
1) A = Z + N
2) N = A - Z
3) Z = A - N
Isotopes are named for their mass numbers. For example, carbon has three naturally occurring isotopes; carbon-12, carbon-13, and carbon-14. The number after the hyphen is the mass number. With this information, we can determine the number of protons (atomic number) and the number of neutrons.
Practice Problem
-
An isotope has a mass number of A =11. The number of neutrons is N = 6. Determine the atomic number and name the isotope.
Answer: Z = A - N = 11 - 6 = 5
Using the periodic table, we see that the element with the atomic number of 5 is boron. isotope is boron-11. -
An isotope of an element has 5 protons and 5 neutrons. Determine its atomic number, Z, and its mass number, A. Name the isotope.
Answer: Z = 5 (because it has 5 protons), N = 5, A = Z + N = 5 + 5 = 10
Using the periodic table, we see that the element with the atomic number of 5 is boron. This isotope's name is boron-10 -
Determine the mass number, atomic number (number of protons), and number of neutrons for carbon-12, carbon-13, and carbon-14.
Answer
3a) carbon-12
Answer: A = 12, Z = 6 (from the periodic table), N = ?
N = A - Z = 12 - 6 = 6
An atom of the carbon-12 isotope has a mass number of 12, an atomic number of 6, and 6 neutrons.
3b) carbon-13
Answer: A = 13, Z = 6 (from the periodic table), N = ?
N = A - Z = 13 - 6 = 7
An atom of the carbon-13 isotope has a mass number of 13, an atomic number of 6, and 7 neutrons.
3c) carbon-14
Answer: A = 14, Z = 6 (from the periodic table), N = ?
N = A - Z = 14 - 6 = 8
An atom of the carbon-14 isotope has a mass number of 14, an atomic number of 6, and 8 neutrons.
Isotopes are frequently written in nuclear or isotope notation. The following diagram shows the way to write an isotope in nuclear notation.
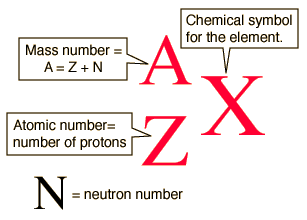
The following diagram shows how to write the carbon isotopes using nuclear notation.
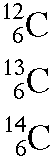
Nuclear Notation Problems
1. Determine A, Z, and N for the following isotopes of oxygen. Then write a statement of what your answer means, similarly to the examples given in the 3rd problem in the previous section.
a)
An atom of oxygen-16 has a mass number of 16, an atomic number (number of protons) of 8, and 8 neutrons.
b)
An atom of oxygen-17 has a mass number of 17, an atomic number (number of protons) of 8, and 9 neutrons.
c)
An atom of oxygen-18 has a mass number of 18, an atomic number (number of protons) of 8, and 10 neutrons.
Notice that in the previous three problems, that you can subtract the subscript from the superscript, which gives you the number of neutrons.
-
Write the following isotopes in nuclear notation.
a) hydrogen-1 Answer:#""_1^1"H"# b) silicon-30 Answer:
#""_14^30"Si"# -
Determine the mass number, A, atomic number, Z, and the number of neutrons, N for each of the following isotopes, and then name the isotope.
a)
Answer: mass number, A = 57, atomic number, Z = 26, number of neutrons, N = 57 - 26 = 31. The name of this isotope is iron-57.
b)
Answer: mass number, A = 96, atomic number, Z = 40, and number of neutrons, N = 96 - 40 = 56. The name of this isotope is zirconium-96.
c)
Answer: mass number, A = 1, atomic number, Z = 1, number of neutrons, N = 1 - 1 = 0. The name of this isotope is hydrogen-1.

