How do you determine the formula of an ionic compound?
1 Answer
Sep 27, 2014
The chemical formula of an ionic compound must have an overall charge of 0. If the cation and the anion do not have equal magnitudes of charge, then subscripts must be added so that the total positive charge and the total negative charge are equal in magnitude. An easy way to do this is to use the criss-cross rule:
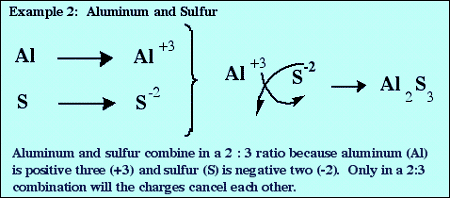
Formulas from Names
a) lead(II) nitride:
b) mercury(I) iodide:
c) hydrosulfuric acid:
d) ammonium phosphate:

