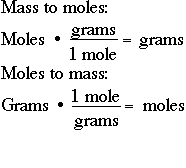How do you convert grams to moles and moles to grams?
1 Answer
Sep 28, 2014
You create a conversion factor from the molar mass and use it in your calculations.
Example 1.
Convert 25.0 g of water to moles of water.
We know that 1 mol H₂O = 180.2 g H₂O.
We can use this to create the conversion factors
We choose the one that gives us the correct units for the answer. So we choose the first one and write
25.0 g H₂O ×
Example 2.
Convert 1.39 mol of water to grams of water.
Here we use the other conversion factor.
1.39 mol H₂O ×