Question #b5738
1 Answer
Oct 7, 2014
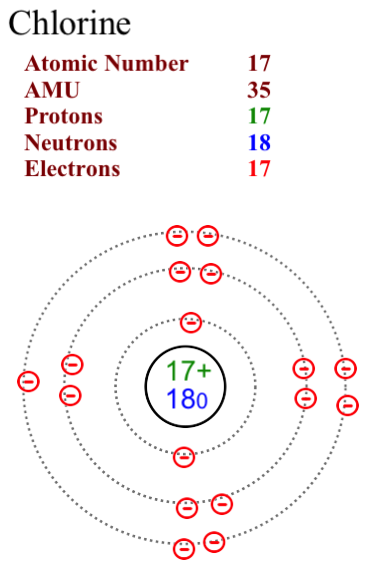
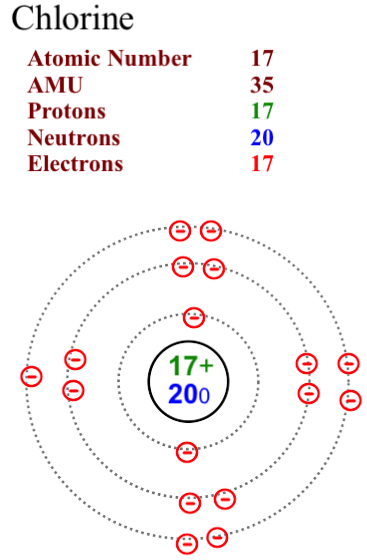
The two most stable isotopes of Chlorine are Chlorine-35 (75.78%) and Chlorine-37 (24.22%).
Isotopes are the various types of atoms of the same element with a different number of neutrons and therefore a different mass. We remind ourselves that the proton number is the same as the atomic number. The element is defined by the proton number. Therefore, the number of protons cannot change without changing the element.
Here are two diagrams of the most common isotopes for chlorine.
Note that in Chlorine-35 the neutron number is 18. In Chlorine-37 the neutron number is 20. The proton and electron numbers remain consistent at 17 as we assume that the atoms are neutral in charge and not ions.