Question #51278
1 Answer
This is a case where there is a possible double replacement reaction, in which the cations and anions switch places and form new compounds. A double replacement reaction is represented by the general equation AB + CD
The products of the reaction between copper(II) nitrate and hydrochloric acid are copper(II) chloride and nitric acid. To determine whether either of these products is insoluble (a solid precipitate), you need to consult a solubility table like the one below:
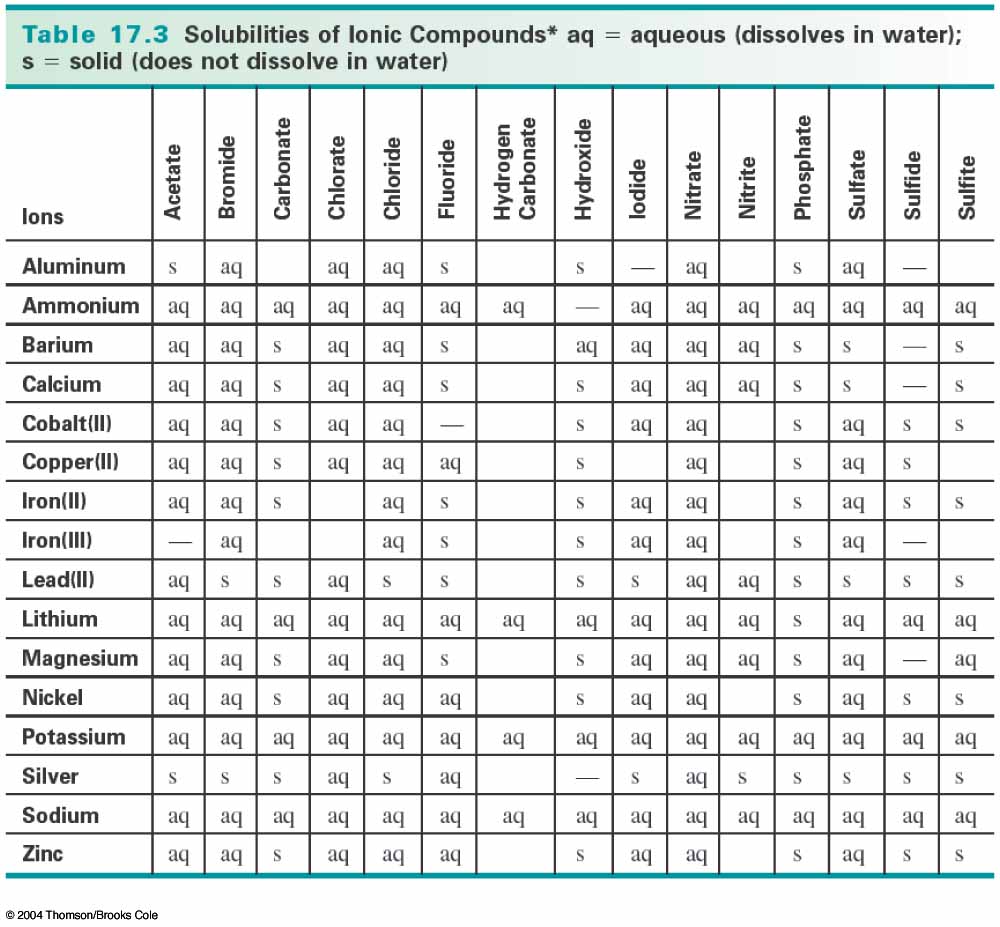
Notice that copper(II) chloride is aqueous, meaning that it is dissolves in water, so it is not a precipitate (solid). Nitric acid is not on the solubility table, however, nitric acid is completely miscible in water, so it is aqueous as well (http://en.wikipedia.org/wiki/Nitric_acid).
The balanced molecular equation for this reaction is:
However, since none of the products are a gas, water, or a precipitate, this reaction does not actually occur, which you would indicate by writing the following:
N.R. means no reaction.

