What type of reaction occurs when barium nitrate reacts with sulfuric acid?
1 Answer
This is a double replacement reaction in which the cations and anions switch places, forming new products. The general form of this reaction is AB + CD
In this double replacement reaction, the precipitate barium sulfate,
The balanced molecular equation for this reaction is:
We can know that barium sulfate is a solid precipitate by consulting a solubility table like the one below:
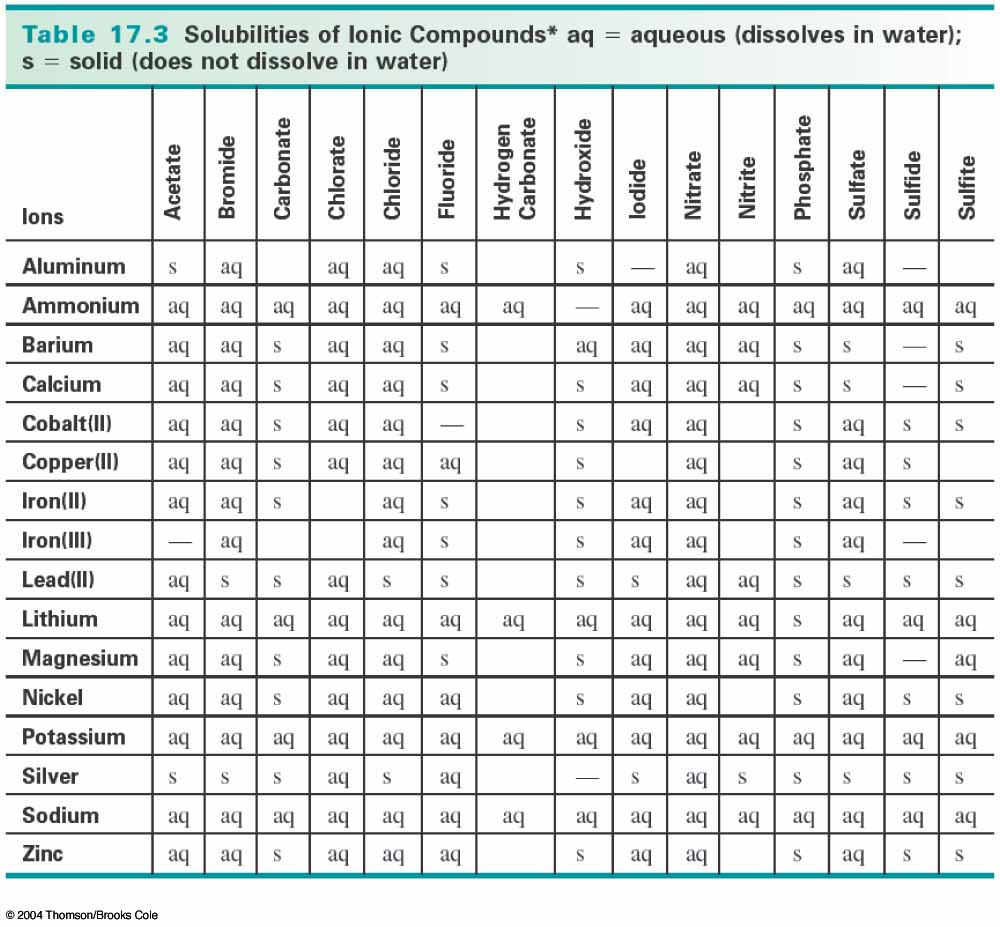
You can see that barium sulfate is listed as "s", which means that it does not dissolve in water, so it precipitates out of solution, forming a solid.
The complete ionic equation , which shows all of the ions and the precipitate is:
The net ionic equation , which shows only those ions involved in the formation of the precipitate, and the precipitate is:
The formula for barium sulfate could also be written
as

