Question #19ae7
1 Answer
The name of the transition metal ion should include the ion charge as a Roman numeral. For example, iron(II) oxide or iron(III) oxide. Iron(II) oxide contains the iron(II) ion,
To find the formula for iron(II) oxide using the crisscross method, the
To find the formula for iron(III) oxide using the crisscross method, the
The crisscross method can be used to determine the formula of any ionic compound, not just the ones with transition metals having more than one charge. The following diagrams will show how to do the crisscross method, but once you get used to it, you can do it mentally.
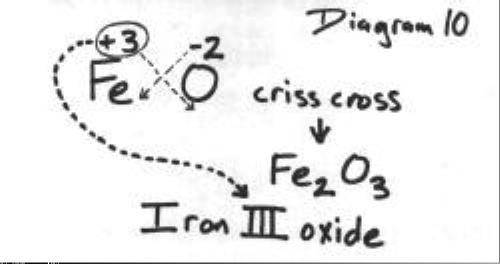
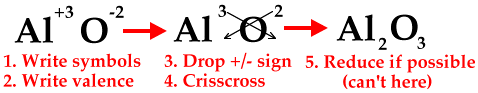
The next diagram shows how to reduce to the lowest whole-number ratio.
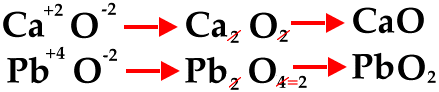
The next diagram shows how to use the crisscross method for polyatomic ions.
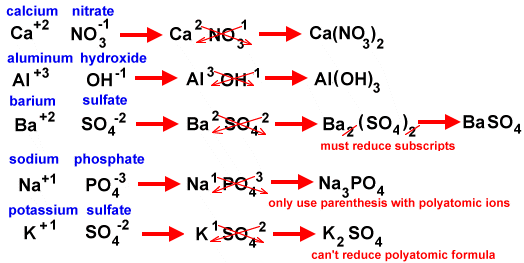
Try copying and pasting the addresses for the diagrams and read through the lessons. The last three diagrams come from the same site.
This You Tube video is very informative and helpful. I hope you'll watch it. There are several other videos under Topics on writing ionic formulas. http://socratic.org/chemistry/ionic-bonds-and-formulas/writing-ionic-formulas/chemistry-53-formula-writing-ionic-compounds
I hope you do well on your test.

