Question #d16ba
1 Answer
Oct 24, 2014
Yes, I can think of two methods for preparing ketones from alkynes.
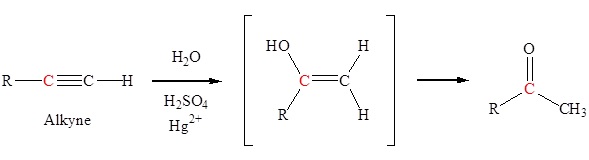
Acid-Catalyzed Hydration
The Markovnikov addition of water to an alkyne, catalyzed by sulfuric acid and Hg²⁺, forms an enol. The enol is unstable and tautomerizes to form a ketone.
Hydroboration-Oxidation of Symmetrical Alkynes
This is a three-step reaction sequence.
1. Addition of boron hydride forms a trialkenylborane.
3RC≡CR + BH₃ → (RCH=CR)₃B
2. Oxidation with basic H₂O₂ forms an enol
(RCH=CR)₃B → 3RCH= CR(OH)
3. The enol tautomerizes to the more stable ketone
RCH= CR(OH) → RCH₂COR

