Are lone pairs of electrons involved in chemical bonding?
1 Answer
Lone pairs of electrons are not involved in chemical bonding. Unpaired electrons are involved in chemical bonding. You can determine lone pairs and unpaired electrons by using Lewis dot structures.
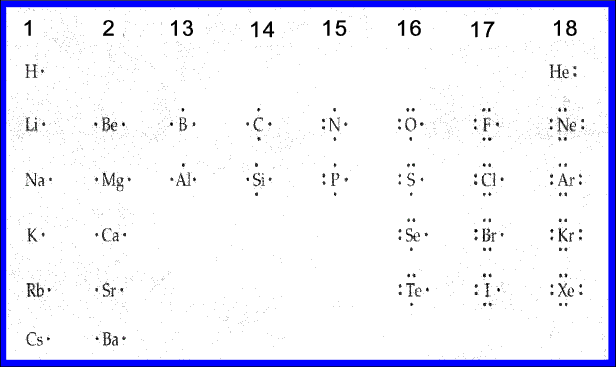
This diagram gives the Lewis dot structures for the main group elements.
All of the elements in groups 1, 2, 13, and 14 lack lone pairs. Their unpaired electrons will be involved in chemical bonding.
The elements in groups 15 through 17 have one or more lone pairs which will not be involved in chemical bonding. However, the unpaired electrons will be involved in chemical bonding.
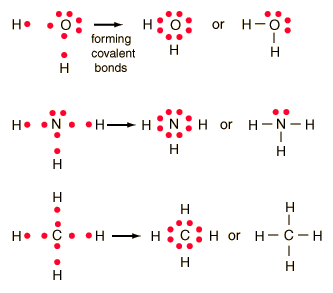
In this diagram, a single bond can be represented by the two dots of the bonding pair, or by a single line which represents that bonding pair. The single line representation for a bond is commonly used in drawing Lewis structures for molecules. By using single lines to represent a bonded pair of electrons, it becomes easier to see the lone pairs of electrons that are not involved in the chemical bonding.

