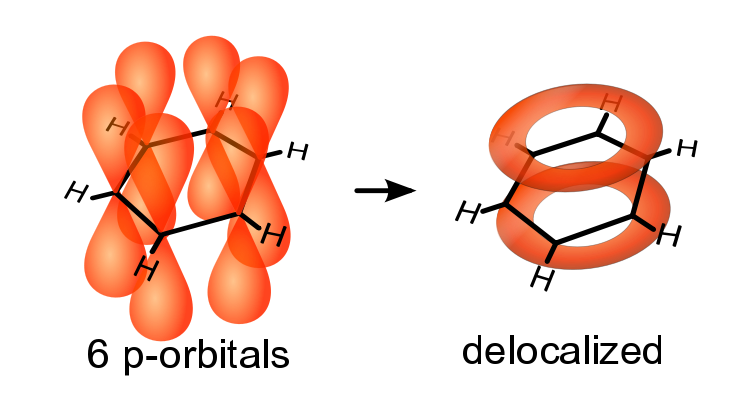What are delocalized electrons?
1 Answer
Nov 19, 2014
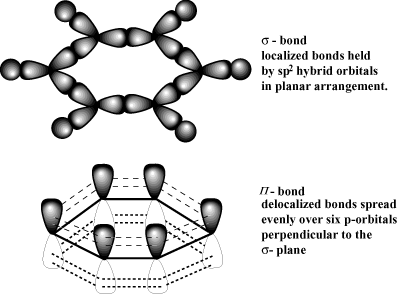
Delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or one covalent bond.
Delocalized electrons are contained within an orbital that extends over several adjacent atoms.
Example: Benzene
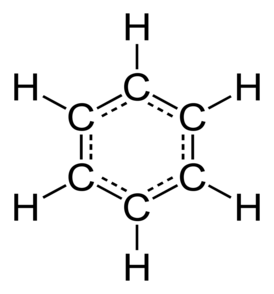
In the simple aromatic ring of benzene the delocalization of six π electrons over the C6 ring is often graphically indicated by a circle or dots.