Question #30c50
1 Answer
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different levels, depending on which element you have. Here are some examples of Bohr diagrams: (These show the atoms lithium, fluorine and aluminum.)
The shell closest to the nucleus is called first orbit or K shell, next to K shell is second orbit or L shell, next to L shell is M shell or third orbit.
Each shell can only hold certain number of electrons. K shell can have 2, L can have 8 , M can have 18 electrons and so on.
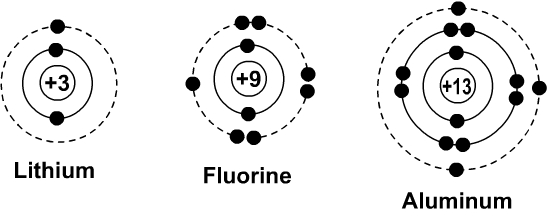
Lithium has three electrons , out of the three electrons , two goes to K shell , remaining one goes to L shell. Its electronic configuration is
K (2) , L (1)
Fluorine has nine electrons , out of the nine electrons , two goes to K shell , remaining seven goes to L shell. Its electronic configuration is
K (2) , L (7) . ( Note L can have 8 electrons)
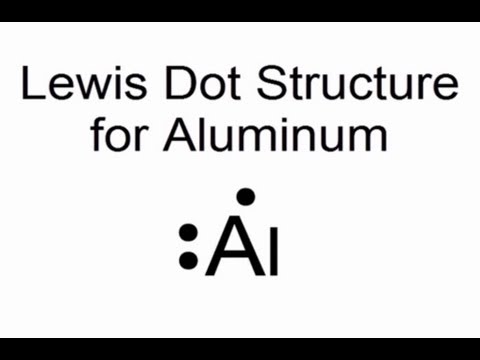
Aluminum has thirteen electrons , out of the thirteen electrons , two goes to K shell , eight goes to L shell, and remaining three goes to M shell. Its electronic configuration is
K (2) , L (8), M (3) ( Note M can have 18 electrons)
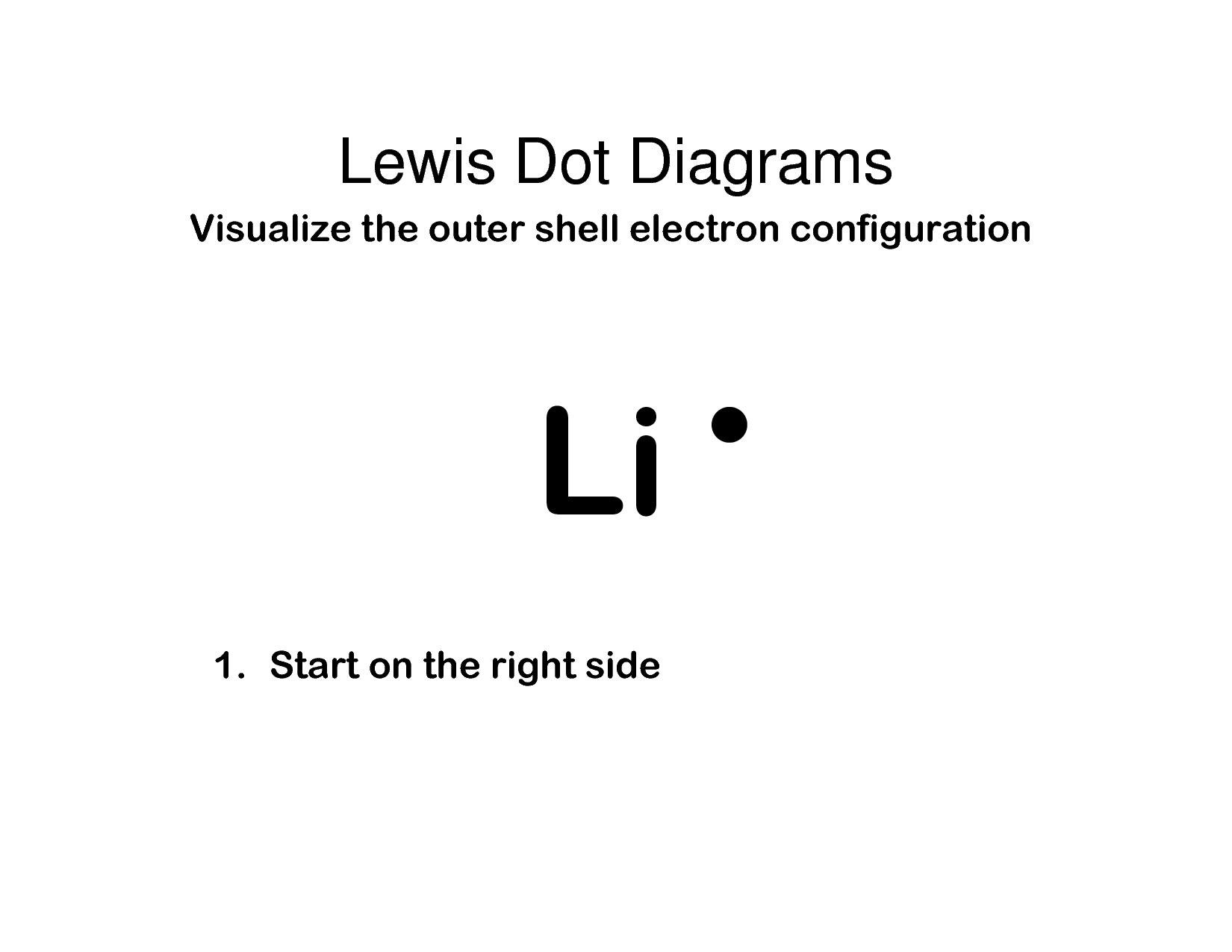
Lewis Symbols are simplified Bohr diagrams which only display electrons in the outermost energy level.
Lithium has one electron in its outermost shell, so its lewis symbol will be
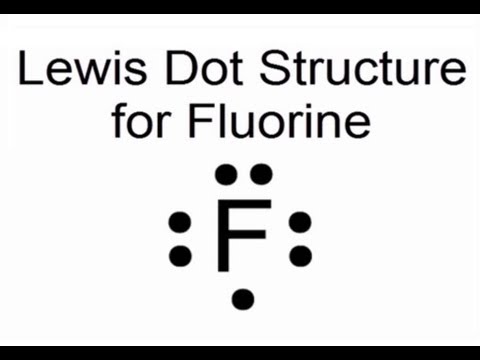
Fluorine has seven electrons in its outermost shell, so its lewis symbol will be
Aluminum has three electrons in its outermost shell, so its lewis symbol will be