Question #587a9
1 Answer
An ion is a charged atom . It is charged because the number of electrons do not equal the number of protons in the atom. An atom can acquire a positive charge if an atom loses one or more than one electrons, An atom can acquire a negative charge if an atom gains one or more than one electrons.

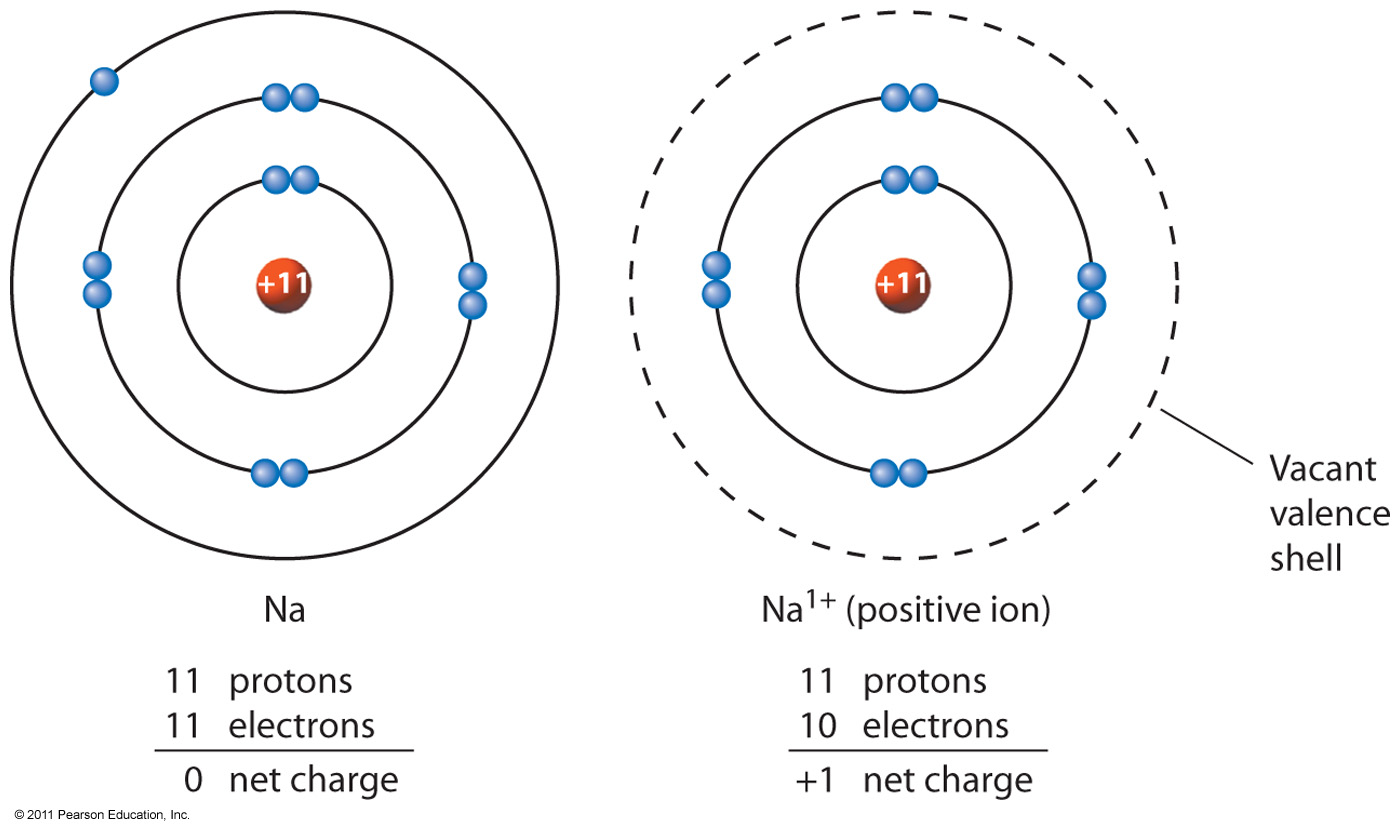
A Sodium atom has 11 electrons and 11 protons. The total positive charge is +11 and total negative charge is -11, overall charge on the atom is 11 + (-11) = 0.
If a Sodium atom loses one electron, it will have 11 protons and 10 electrons. The total positive charge is +11 and total negative charge is -10, overall charge on the atom is 11 + (-10) = +1.
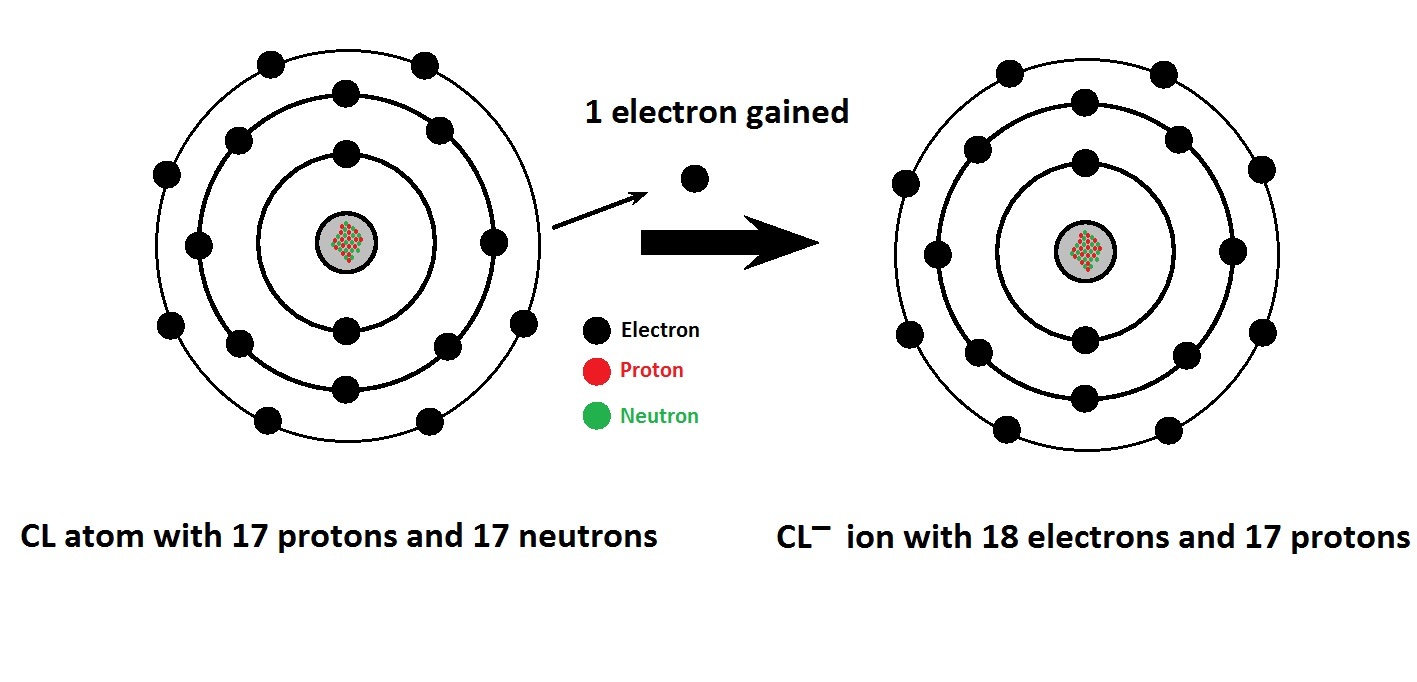
A Chlorine atom has 17 electrons and 17protons. The total positive charge is +17 and total negative charge is -17, overall charge on the atom is 17 + (-17) = 0.
If a Chlorine atom gains one electron, it will have 17 protons and 18 electrons. The total positive charge is +17 and total negative charge is -18, overall charge on the atom is 17+ (-18) = -1.