What determines whether a solid is soluble in water?
1 Answer
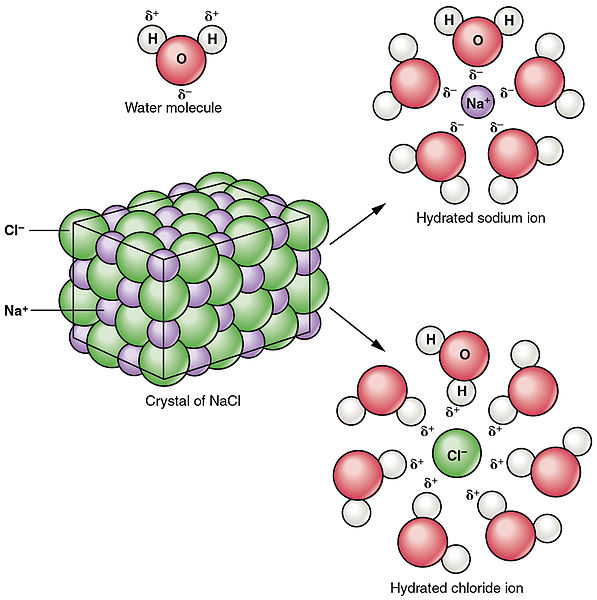
Whether a solid is soluble in water depends on its polarity. Since water is a polar molecule, it will only dissolve polar solids, and many ionic compounds which dissociate in water. Water does not dissolve nonpolar molecular compounds, and does not dissolve all ionic compounds.
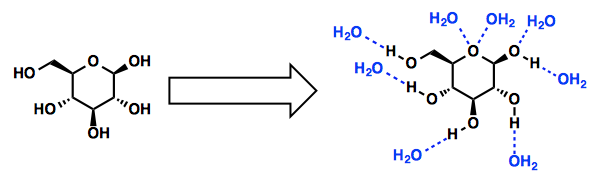
Glucose is a polar molecule that contains many OH groups which break the O-H hydrogen bonds between water molecules, and form hydrogen bonds between the H in their OH groups and the partially negatively charged oxygen atoms in water molecules.
Sodium chloride, an ionic compound, dissociates into individual