Question #b736b
1 Answer
We can solve this by making a table as follows:
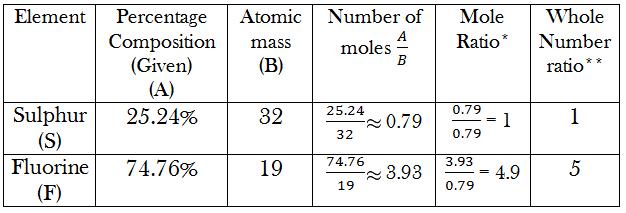
*Calculation of Mole Ratio: Divide the number in the previous cell by the lowest value in the previous column. In this case, the lowest value in the 4th column is 0.79, so every number should be divided by 0.79.
**Calculation of Whole Number Ratio: Round off the number obtained in the previous cell to the closest whole number.
Now that we have obtained the whole number ratios of the element, we can find the Empirical Formula. There are 5 atoms of fluorine for every atom of sulphur.
Empirical Formula:
Now,
Molar mass of the compound = 254.1 g/mol
The molecular formula can be calculated by multiplying the empirical formula by the ratio
Note: Empirical Formula Mass can be calculated using the Empirical Formula. In that, there is one sulphur atom and five fluorine atoms. Thus, the empirical formula mass
Therefore,
Molecular Formula = (Empirical Formula) ×
Thus, the empirical formula of the compound is
I hope I was able to help!
