Question #9b2bd
1 Answer
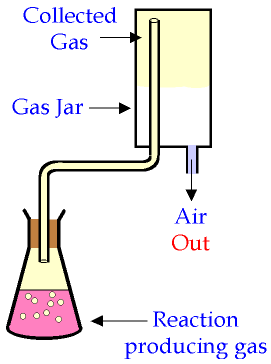
If a gas is less dense than air, it is often more convenient to collect it in a gas jar or test tube by upward delivery. The gas produced in a chemical reaction is passed through a delivery tube into the gas jar, where it rises and takes up the space at the top of the jar - pushing the air in the jar down, and out at the bottom.
This works well for hydrogen and ammonia, which are both less dense than air.
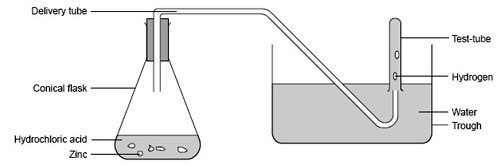
Sometimes gases are collected over water. The gas produced in a reaction is bubbled through a trough of water and into an upturned gas jar filled with water. The bubbles of gas collect in the top of the gas jar and push the water out of the bottom. If enough gas is produced it completely replaces the water in the gas jar. A glass lid is then slid under the gas jar, which is then removed from the trough of water and turned the right way up.
This works well for insoluble gases such as hydrogen, or gases that do not dissolve easily in water, such as oxygen and carbon dioxide. Ammonia and chlorine are readily soluble in water and are not collected this way.