How can I classify each species as either a Lewis acid or a Lewis base for #BeCl_2#, #OH^-#, #B(OH)_3# and #CN^-#?
1 Answer
You draw their Lewis structures and then make a decision based on possible reactions.
A substance may be either a Lewis acid or a Lewis base, depending on the reaction. But you can often make a good prediction.
BeCl₂
The Lewis structure for BeCl₂ is

We see that the Be atom has only four electrons in its valence shell. Be has an incomplete octet.
The Be atom will accept electrons to complete its octet.

So BeCl₂ is a Lewis acid.
OH⁻
The Lewis structure of OH⁻ is
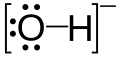
The O atom has three lone pairs of electrons. It can donate these electrons to form a bond to another atom.

OH⁻ is a Lewis base.
B(OH)₃
The Lewis structure of B(OH)₃ is

The B atom has an incomplete octet, so it can act as a Lewis acid.
HO⁻ + B(OH)₃ → [HO-B(OH)₃]⁻
B(OH)₃ is also an oxyacid. So the H atom can also act as a Lewis acid.
HO⁻ + H-O-B(OH)₂ → H₂O + ⁻O-B(OH)₂
However you look at it, B(OH)₃ is a Lewis acid.
CN⁻
The Lewis structure of CN⁻ is
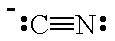
The C atom has both a lone pair and a negative charge, so it will behave as a Lewis base.
N≡C:⁻ + CH₃-Br → N≡C-CH₃ + Br⁻
Here’s a video on how to identify Lewis acids and bases.

