Question #671ba
1 Answer
The explanaition for this phenomenon is given by Raoult's law; according to this, when a nonvolatile solute is dissolved in a solvent, it lowers the vapor pressure of the solvent (vapor pressure lowering) because of the interactions between solvent and solute molecules.
At this time, solvent to solute attractions are what will cause less solvent particles to evaporate, often times because there are simply less solvent molecules at the surface, and because solvent molecules now need more energy to escape into gas form.
Raoult's law states that for an ideal solution, the partial pressure of a component present in that solution is equal to the mole fraction of that component times its vapor pressure when pure.
Now, it's onvious that when
However, when
Here is a representation of this for
The first gif represents pure water, while the socond one represents the solution:
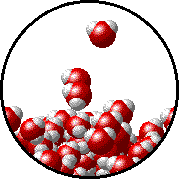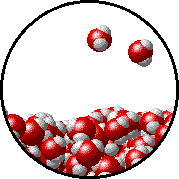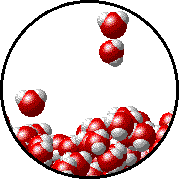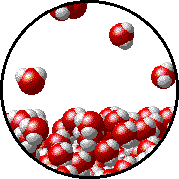
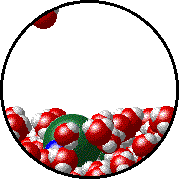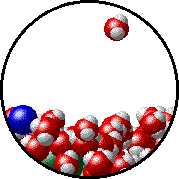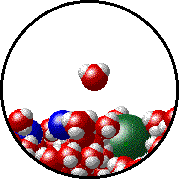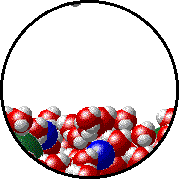
Notice how the intermolecular forces between the water and the solute particles (in this case,

