How do I calculate vapor pressure of water?
1 Answer
You could use Antoine's equation to calculate the vapor pressure of water. Antoine's equation is a vapor pressure equation that describes the relationship between vapor pressure and temperature for pure components. So,
You'd get
This equation is usually used with two sets of paramaters, a low-pressure paramater that describes the vapor pressure curve up to the normal boiling point, and a second paramater that covers the range from boiling point to critical point.
So, for water, the two sets of paramaters are
SET 1.
SET 2.
Notice that you can use both sets of paramaters for temeperatures between
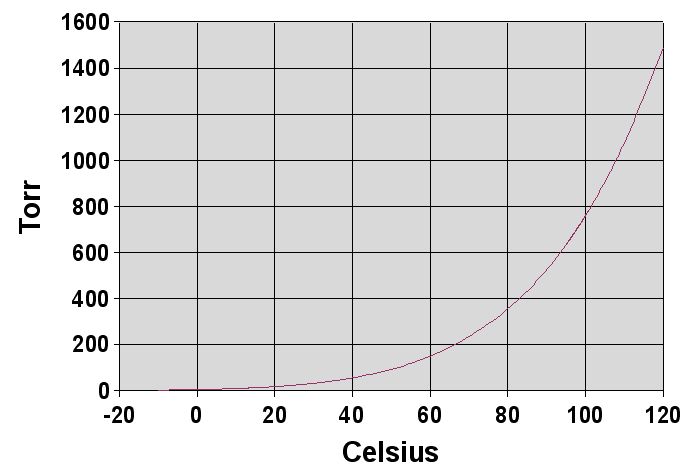
Here's a diagram of what an Antoine curve would look like