The first step in drawing the Lewis dot structure for ethane (C_2H_6) is to determine how many valence electrons are available for the molecule.
Since C has 4 valence electrons, and each H atoms contributes 1 valence electron, the total number of electrons will be
2*4 + 6*1 = 14 "e"^(-)
This means that the Lewis dot structure for C_2H_6 must account for 14 valence electrons, either through bonding between atoms, or through lone pairs.
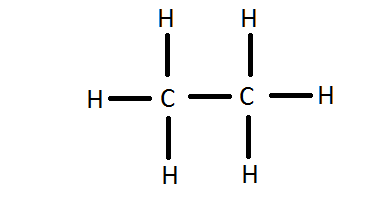
So, the two C atoms are placed in the center of the molecule. Each C atom forms three covalent bonds with three H atoms, with one aditional covalent bond being formed between the two C atoms.
Each of these seven single bonds contains 2 electrons, which means that a total of
7 * 2 = 14 "e"^(-) were used for the C_2H_6 molecule.


