What is an example of a nuclear equations practice problem?
1 Answer
The two most common types of problems you'll see in nuclear chemistry involve either nuclear half-life calculations, or balancing nuclear equations.
I'll show you an example on how nuclear equations pop up in exams or tests. More often than not you will be asked to complete a certain nuclear equation and name the reaction, like
When balancing nuclear equations It is very important to know that the sum of the atomic masses must be equal on both sides of the equation; likewise, the sum of the atomic numbers must be equal on both sides.
An isotope's atomic mass is represented by the top number, while its atomic number is represented by the bottom number.
In the above example,
So, we know that matter must be conserved in any type of nuclear equation - this includes both protons and neutrons, of course. Let's take the first stage of this equation
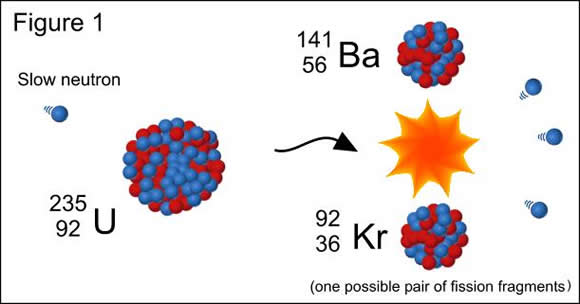
Here, an U-235 isotope is bombarded with a neutron - notice that the neutron has no charge - the bottom number is 0 - and a mass of 1 - the top number. SInce the atomic number will be unchanged, we know for sure that we are dealing with another uranium isotope, but this time its atomic mass will be
Now for the second stage
Here, two elements, which are called daughter nuclei fragments, are formed. Let's solve for the missing particle's atomic mass and atomic number
We get that the particle's atomic number is 0 and that its atomic mass is 3, which implies that we are dealing with not one, but three neutrons emitted in the second stage. So, the balanced nuclear equation is
This particular nuclear equation describes the formation of a possible pair of fission fragments for the fission of uranium-235.