The formation of CoCl4 2- from Co 2+ and Cl- is endothermic. Are the color changes that accompany heating and cooling of equilibrium mixture in accord with Le Chatelier's principle?
1 Answer
Yes, the color changes that accompany the heating or cooling of the equilibrium mixture are very much in accordance with Le Chatelier's Principle. Here's what actually goes on.
The main species involved in the reaction is cobalt (II) chloride, or
In aqueous solution, the following equilibrium will be established between the following two ions:
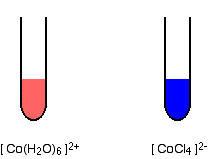
The
We can say that heat is a reactant in this equilibrium. This means that when heat is added, i.e. the solution is being heated, the equilibrium will shift in the direction of the products. This shift in the equilibrium will turn the solution blue, the color of the
When the solution is being cooled, heat is being removed from the equilibrium, which means that a shift towards to the reactants will take place - the solution will turn pink, the color of the
Likewise, any other stress applied to the equilibrium will produce a color change in the solution. If you add water, the equilibrium will shift to the left and the color will be pink. If you add
If you add
So, as you can see, all these changes are undoubtedly in accordance with Le Chatelier's Principle.
Here's another video on this great lab experiment:

