Question #ce59d
1 Answer
An element's mass number represents the number of protons and neutrons it has in its nucleus.
A quick example:
http://www.chemteam.info/AtomicStructure/Nuclear-Symbol.html
Helium's mass number is 4, meaning that the number of protons plus the number of neutrons it has in its nucleus is 4. Since helium has an atomic number of 2, which expresses the number of protons it has in its nucleus, you can deduce that it will have
Another example of an element's mass number is carbon
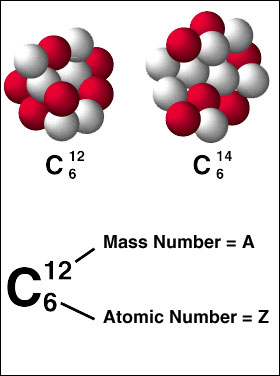 http://community.dur.ac.uk/foundation.science/?q=blog&page=43
http://community.dur.ac.uk/foundation.science/?q=blog&page=43
Notice that the

