Why is the propane that used as a fuel in a BBQ gas at room temperature, but 2-propanol used as rubbing alcohol is a liquid at room temperature?
1 Answer
The obvious answer is because propane is a gas at room temperature, while 2-propanol is a liquid at room temperature.
That happens because the intermolecular forces that exist between 2-propanol, or isopropyl alcohol, molecules are stronger than those that exist between propane molecules.
Propane is a hydrocarbon, which means it only has
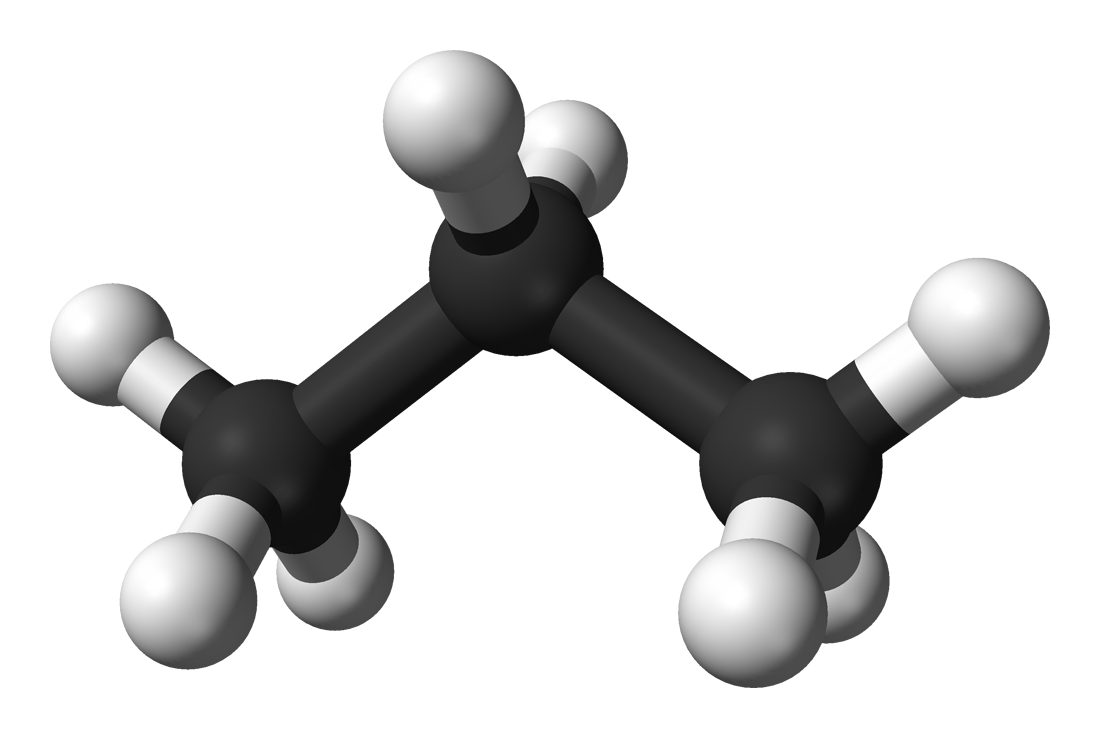
As a result, the propane molecule will be nonpolar, since no separation of charge will exist, i.e. the molecule has no permanent dipole moment. The direct consequence is that it only exhibits weak London dispersion forces, also known as Van der Waals interactions.
On the other hand, 2-propanol, like its name suggets, is an alcohol, which means it has the
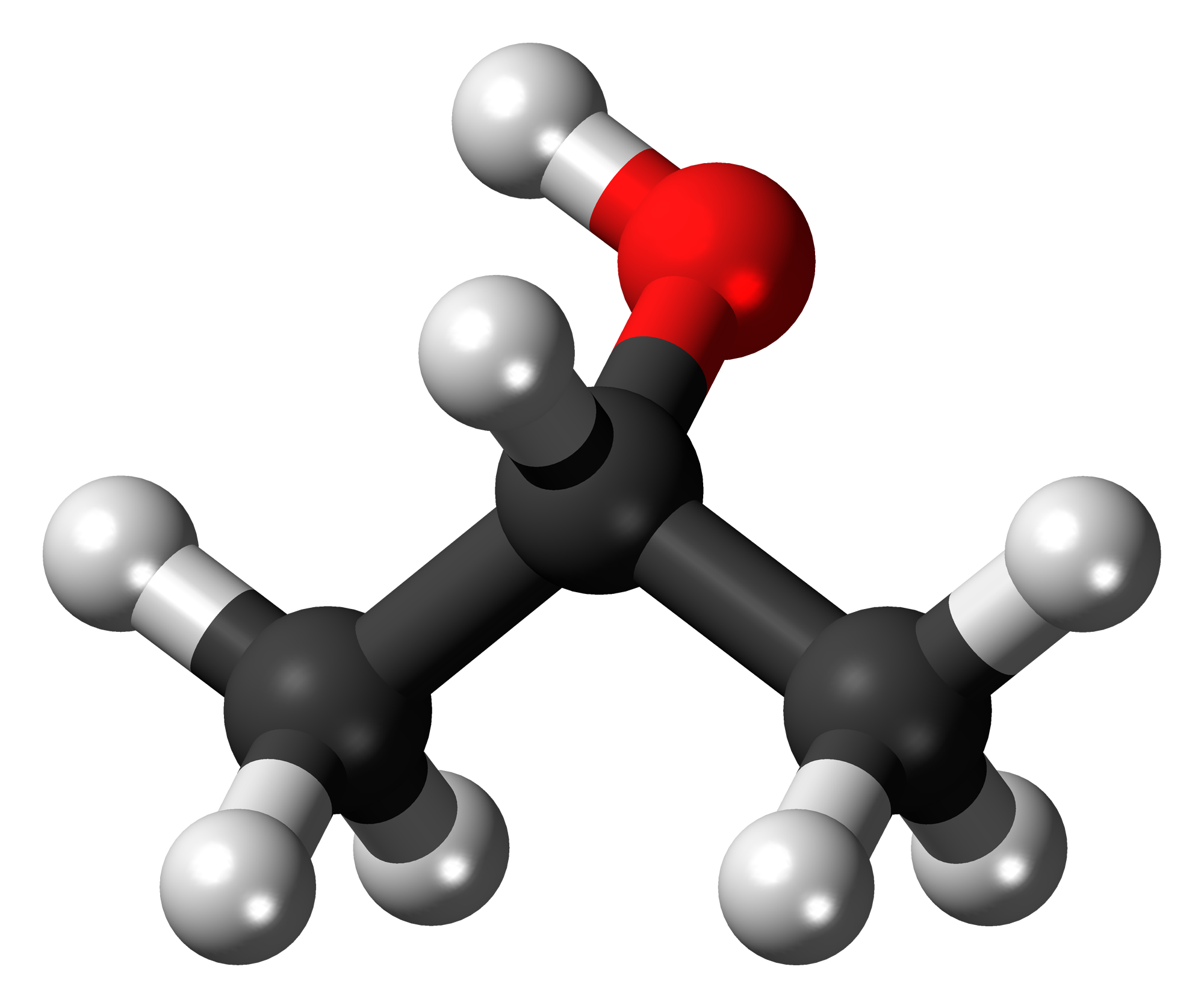
In the above picture, the oxygen atom is shown in red (carbon atoms are shown in black and hydrogen atoms in grey). The addition of the
The molecule will now be polar, since a permanent dipole is established by the presence of the more electronegative oxygen atom. This means that it will exhibit stronger dipole-dipole interactions.
Moreover, the molecule has the ability to form hydrogen bonds. the strongest intermolecular forces, because it has a hydrogen atom bonded to an oxygen atom.
Stronger intermolecular forces mean a lower vapor pressure and a higher boiling point for 2-propanol than for propane.
By comparison, propane boils at

