Question #b0544
1 Answer
Water molecules are able to form hydrogen bonds with C) compounds that have polar covalent bonds.
Water molecules can only form hydrogen bonds between the partially positively charged hydrogen atoms in a water molecule and a partially negatively charged atom in another molecule. This means that hydrogen bonds can only form between atoms in polar covalent bonds.
Answer Explained:
A) The bond between the O atoms in a molecule of oxygen gas is nonpolar, which means the atoms in a molecule of oxygen gas are not partially charged, so they cannot form hydrogen bonds with water molecules; B) compounds that are not soluble in water are nonpolar and their atoms do not form partial charges; and D) oils are also nonpolar. Therefore, they would not be able to form hydrogen bonds with water molecules.
The image below shows hydrogen bonding between water molecules. The symbol
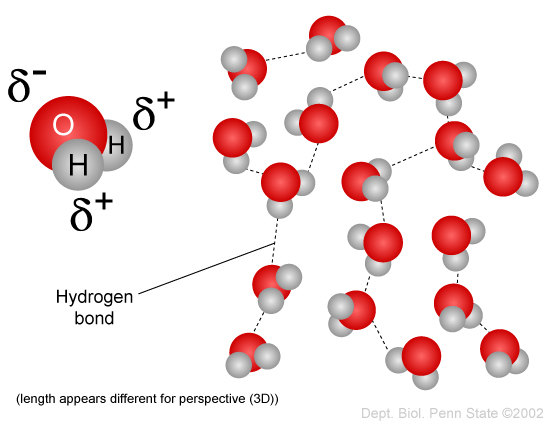
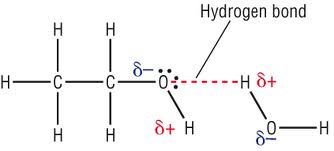
Water molecules can form hydrogen bonds with a variety of molecules, as long as they have one or more polar covalent bonds. The following image shows hydrogen bonding between the polar H atom of the water molecule and the polar O atom of ethanol,
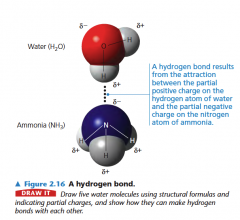
Hydrogen bonding can also occur between a partially negatively charged nitrogen atom in a molecule of ammonia. The partially charged hydrogen atoms in ammonia could also form hydrogen bonds with the partially negatively charged oxygen atoms in the water molecules.
The ability of water molecules to form hydrogen bonds with other molecules that have polar covalent bonds is the basis for the dissolution of these compounds in water. Remember the saying, "Like dissolves like."

