Is baking soda an ionic compound?
1 Answer
Feb 20, 2015
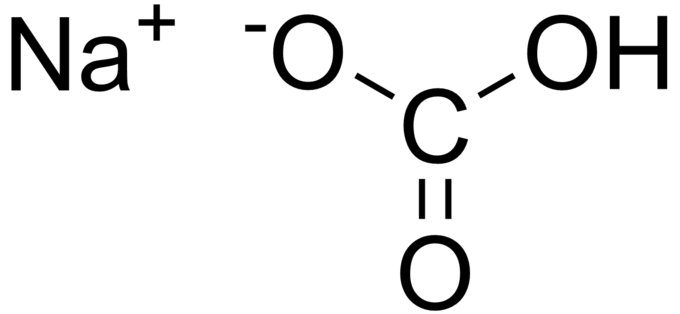
Yes, baking soda is an ionic compound. Baking soda is composed of sodium ions,
Commercial quantities of baking soda are produced by the following method: soda ash (
http://en.wikipedia.org/wiki/Sodium_bicarbonate
The following is a structural diagram of the compound.