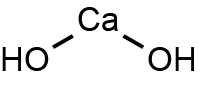
What is the chemical formula of calcium hydroxide?
1 Answer
Feb 22, 2015
Calcium hydroxide is formed by one
Here's how you'd figure this out. If you look at the periodic table, you'll see that calcium is in group 2. That means that it has two valence electrons that it must give up in order to be stable.
On the other hand, oxygen is in group 6, which means it needs 2 electrons in order to be stable. It gets one electron from a hydrogen atom to form an
This means that you'd need two