How could IR spectroscopy distinguish between #CH_3CH_2CH=CHCH_3# and #CH_3CH_2C≡C CH_3#?
1 Answer
A peak at about 3020 cm⁻¹ indicates an alkene, while a peak near 2100 cm⁻¹ indicates an alkyne.
The characteristic IR frequencies of alkenes are:
- C=C stretch between 1640-1680 cm⁻¹
- =C-H stretch between 3000-3100 cm⁻¹ (above 3000 cm⁻¹!)
- =C-H bend (cis) near 700 cm⁻¹
- =C-H bend (trans) near 970 cm⁻¹
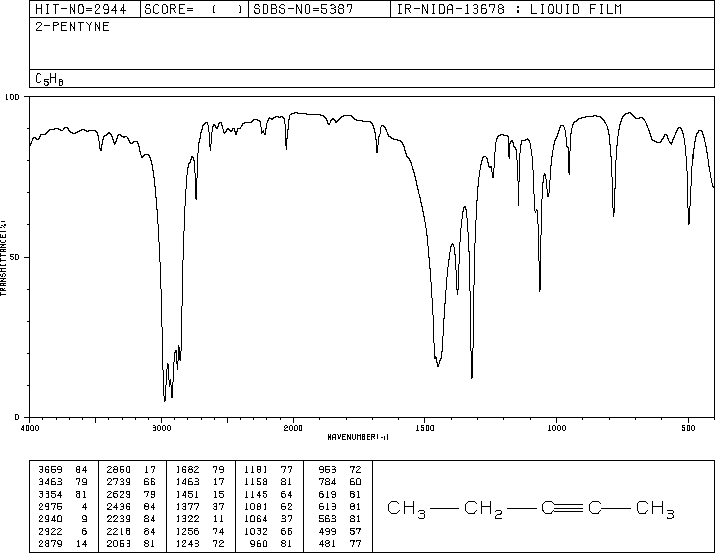
The characteristic frequency of an internal alkyne is a sharp, weak C≡C stretching band near 2100 cm⁻¹.
The IR spectrum of cis-pent-2-ene and trans-pent-2-ene are shown below as spectra B and C.


They each have peaks at about 3020 cm⁻¹.
The C=C stretch of the cis isomer is near 1660 cm⁻¹.
The corresponding peak for the trans isomer is almost undetectable because of the symmetry of the C=C bond. There is almost no change in dipole moment as the bond stretches.
But you can distinguish the two isomers because cis has a C-H bending vibration near 700 cm⁻¹. For trans, the C-H bend is near 960 cm⁻¹.
The IR spectrum of pent-2-yne has a sharp, weak peak near 2100 cm⁻¹.