When a cook heats water to make pasta, energy flows _____ the pot of water, so DH is _____. A) into, 0 or B) into, > 0 ?
1 Answer
Mar 2, 2015
The answer is B) into, > 0.
I'm assuming you had more options and narrowed them down to these two, since you're basically stuck with "into" as one of the blanks.
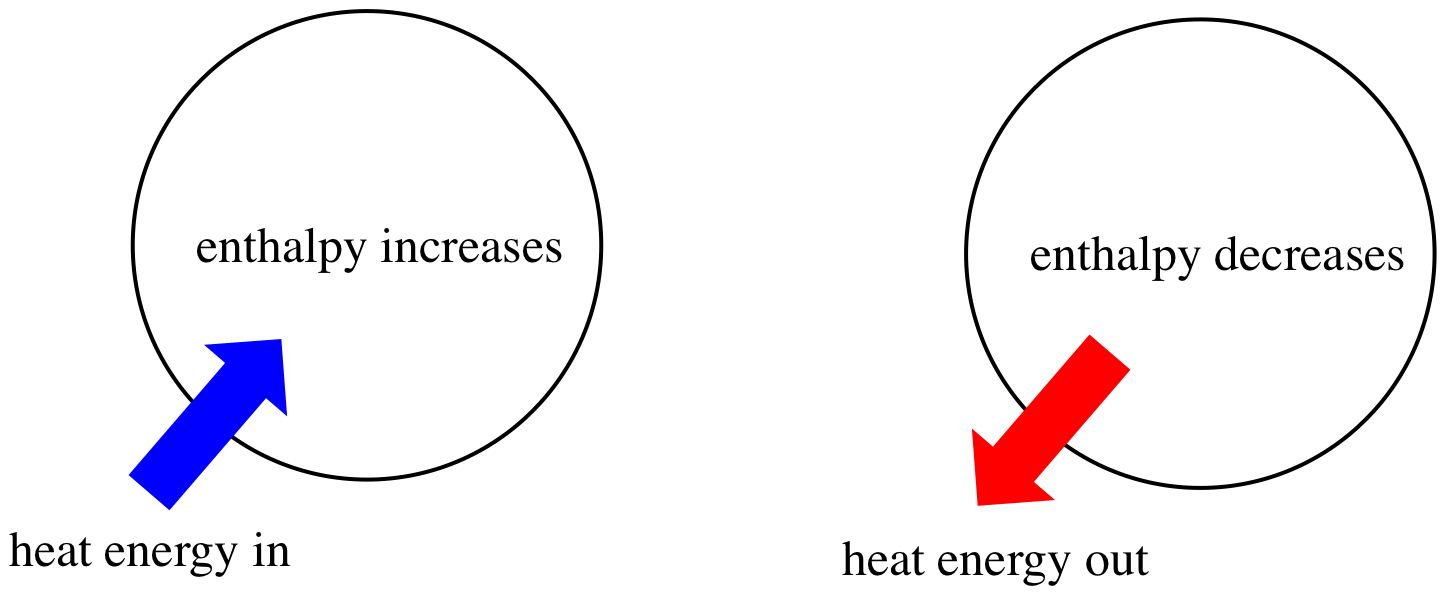
If by DH you mean
Enthalpy changes are always considered from the system's point of view. Energy is being transferred from the stove into the cooker, so, from water's perspective, energy is being put into it.
Water's gaining energy, so its enthalpy change is positive.