Question #465e4
1 Answer
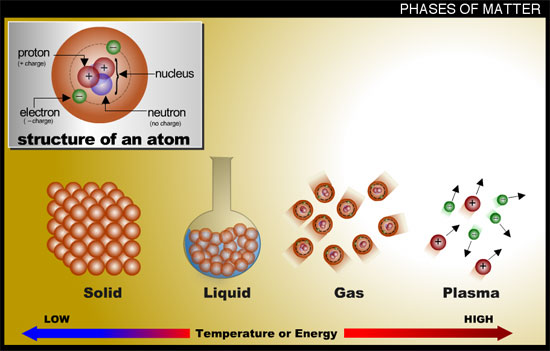
Matter is classified by the arrangement of the particles in space and by their motion.
Solids have particles that are close together and held tightly with ionic or covalent bonds. Crystals with ionic bonding are the most ordered. The particles in a solid can only vibrate back and forth.
Liquid particles have more kinetic energy than solids so even though they are close together they have enough energy to flow around each other. Liquids take the shape of the container you pour them in.
Gas particles are far apart compared to solids and liquids. Gas particles move constantly with rapid, random motion. They collide with each other and with the walls of the container you put them in.
Gases take the shape and size of their container.
Plasma is described as a super heated gas with so much energy that the positively and negatively charged particles become very far apart.
Plasmas are ionized gases without defined shape or volume.