How do you write the nuclear notation of an isotope?
2 Answers
Usually the symbol is written first, then the mass number superscripts to the top left and the atomic number subscripts to the bottom right.
This question is vague. I would need more information to give you an appropriate answer.
Since you didn't identify which isotope, I'm going to use carbon-14 as an example. An isotope is named for its mass number, which is the sum of the protons and neutrons in the nucleus. For this reason, isotopic notation is also known as nuclear notation. Carbon-14 has an atomic mass of 14. When writing isotopic/nuclear notation, the symbol of the element is written with the mass number as a superscript on the left, and the number of protons (atomic number) as a subscript on the left. The following diagram shows the proper format.
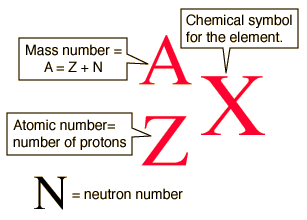 http://hyperphysics.phy-astr.gsu.edu/hbase/nuclear/nucnot.html
http://hyperphysics.phy-astr.gsu.edu/hbase/nuclear/nucnot.html
If you know the mass number, A, and the atomic number (protons), Z, you can determine the number of neutrons (N) by subtracting Z from A (A - Z = N).
Back to carbon-14. On the periodic table, we can see that carbon's atomic number is 6, so it's number of protons is 6. This will be the subscript. Its mass number is 14. This will be the superscript. So the isotopic/nuclear notation for carbon-14 is:


