Question #23703
1 Answer
No, sulfur is indeed in period 3, (main) group 6 of the periodic table.
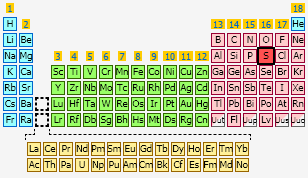
From the top of the periodic table, you descend 3 periods, or horizontal rows, to get to period 3.
If you skip transition metals altogether, this period contains sodium, or
SIDE NOTE The group in which sulfur is lcoated in actually group 16. An alternative way of naming the groups has sulfur in group VIA, or main group 6.
This method skips transition metals (showed in green and yellow in the above image of the periodic table) in naming main groups.
So, you can give sulfur's group number as either 16, or VIA.
The original statement is correct, sulfur is in period 3, (main) group 6 of the periodic table.

