Question #5b62e
1 Answer
The 2 subscript used for diatomic oxygen, or
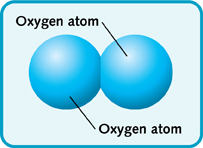
In other words, you need two oxygen atoms to form one oxygen molecule.
Because two oxygen atoms are used to form one oxygen molecule, the molar mass of the molecule will be twice that of an individual oxygen atom.
There is a very important distinction to be made between molecules and compounds. A molecule consists of two or more atoms that can either be identical or distinct.
Compounds, on the other hand, are substances comprised of two or more elements, or atoms, that cannot be all identical.
For example, something like
By comparison, water, or
So,


