Question #67926
2 Answers
The periodic table of elements is an amazing classification tool. There are many things that we chemists do which only require a quick glance at the table to obtain the answer we require.
The periodic table is the arrangement of the elements in increasing atomic number.
The modern periodic law: Properties of elements are periodic functions of their atomic numbers.
Simply put the properties of elements are dependent upon their atomic numbers and these properties repeat themselves after a certain period.
Its made up of horizontal rows called PERIODS and vertical columns called GROUPS.
Each PERIOD starts with an alkali metal and ends with a noble gas.
Its read left to right.The period number=number of orbit/s in the atom of the elements of the period.
Each group has a number and this number = number of valence electron/s in the members of the group.All the group members have same number of valence electrons,therefore their properties are same ( only proportionally stronger or weaker)
Its read up to down(preferably) / down to up
There are many trends that coincide with the order of the periodic table.
1. Atomic Size/Radius: Its the distance between center of nucleus and the outermost orbit.
Across Period: The atomic no increases
(the trend is different for ions.) !
2.Ionization Energy (the energy required to remove and electron from the outermost orbit of an element.)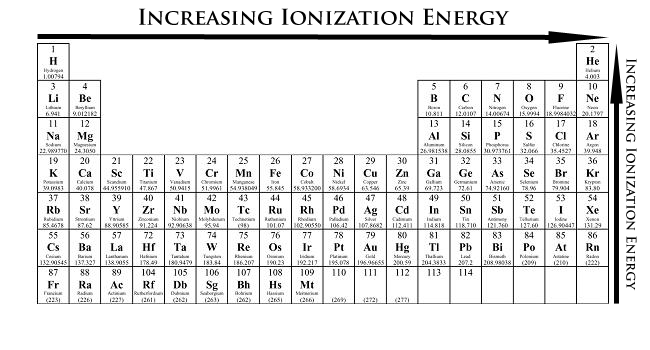
Across period: as atomic size decreases with increasing nuclear pull the ionization energy increases.
Down the group: with increasing atomic size and decreasing nuclear pull the ionization energy decreases.
There are many trends. But when you understand and use the logic explained above. many of them are easy to understand.some of others are
3.Electron affinity: The energy given out when an electron is added to outermost orbit of an atom.
Across period: as atomic size decreases with increasing nuclear pull the Electron affinity increases.
Down the group:with increasing atomic size and decreasing nuclear pull the Electron affinity decreases.
get the drift :)
4.Electronegativity (a number that helps us understand the relative strength of attraction an atom has for electrons.) or the tendency of an element to attract electrons towards itself in a co-valent bond chemwiki.ucdavis.edu
And there are even more where these came from.
I hope that all of this helps you to understand that the periodic table isn't just important, it is one of the most important tools that we have.
The Periodic Table is a way of listing the elements.
- Elements are listed in the table by the structure of their atoms.
- This includes how many protons they have as well as how many electrons they have in their outer shell.
- From left to right and top to bottom, the elements are listed in the order of their atomic number, which is the number of protons in each atom.
Each element has its own name and abbreviation in the periodic table. Some of the abbreviations are easy to remember, like H for hydrogen. Some are a bit harder like Fe for iron or Au for gold.
-
The elements in group 1 are known as alkali metals. They react rapidly (very fast) with water, producing an alkaline solution and hydrogen gas. The metals become more reactive as you go down the group.
-
The elements in group 7 are known as halogens. Fluorine and chlorine are gases. Bromine is one of only two liquid elements. Iodine is solid. They exist as diatomic molecules - they have two atoms in each molecule. As you go down the group the halogens become less reactive.
-
The elements in group 0 are known as noble gases. They are very unreactive and exist as individual atoms (monatomic).
-
The transition metals are elements which are found between groups 2 and 3. Well known examples are iron, copper and gold. They are generally quite dense (heavy) and many form brightly coloured compounds.
http://www.bbc.co.uk/bitesize/standard/chemistry/elementsandreactions/periodic/revision/2/



