Question #83f42
3 Answers
Please tell me that you recorded the amount of vinegar that you put inside your Erlenmeyer flask or container prior to titration.
So there would be 3 different volumes of vinegar on your 3 different containers.
Those are important in computing for the molarity of the vinegar.
so please indicate first all the volume
The average molarity of the acetic acid is 0.8 M.
The balanced equation for the reaction is
HA + NaOH → NaA +H₂O
The moles of acetic acid in in each titration are given by the formula:
The molarity of acetic acid comes from the formula
Your experimental data were
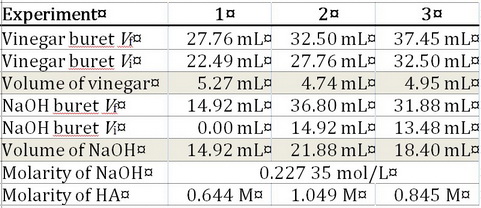
They don't make sense, because the volume of NaOH decreases as the volume of acetic acid increases.
Here are the calculations for Experiment 1.
The average molarity is
The answer can have only 1 significant figure because your numbers all vary in the first decimal place.
Vinegar contains about 5 % by mass of acetic acid (50 g/L or 0.8 M). Your result is therefore accurate but not precise.

