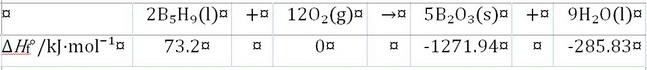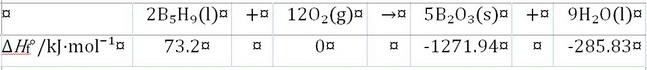We have the following information.
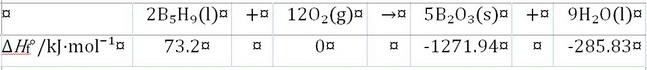
#ΔH_"rxn"^° = ΣΔH_"f"^°("p") - ΣΔH_f^°("r")#,
where #"p"# = products and #"r"# = reactants.
#ΣΔH_"f"^°("p") = (5 cancel("mol B"_2"O"_3) × "-1271.94 kJ"/(1cancel("mol B"_2"O"_3))) + (9 cancel("mol H"_2"O") × "-285.83 kJ"/(1cancel("mol H"_2"O"))) = "-8932.17 kJ"#
#ΣΔH_f^°("r") = (2 cancel("mol B"_5"H"_9) ×"73.2 kJ"/(1cancel("mol B"_5"H"_9))) + (12 cancel("mol O"_2) × "0 kJ"/(1cancel("mol O"_2))) = "146.4 kJ"#
#ΔH_"rxn"^° = ΣΔH_"f"^°("p") - ΣΔH_f^°("r") = "(-8932.17 - 146.4) kJ" = "-9078.57 kJ"#,
But this is the energy released by 2 mol of #"B"_5"H"_9#.
#2 cancel("mol B"_5"H"_9) × ("63.13 g B"_5"H"_9)/(1 cancel("mol B"_5"H"_9)) = "126.26 g B"_5"H"_9#
So #ΔH_"rxn"^"o" = "-9078.57 kJ"/("126.26 g B"_5"H"_9) = "-71.90 kJ/g B"_5"H"_9"#