Why do the energy levels in an atom have negative energy values?
1 Answer
Mar 23, 2015
I can give you the student version that I got when I was studying the hydrogen atom;
Basically the electron is bound to the atom and to free it from the atom you must "give" energy to the atom until the electron reaches a level of zero energy. At this point the electron is neither free nor bound (it is in a kind of "limbo"!). If you give a little bit of energy the electron acquires it (so now it has "positive" energy) and flies away! So when it was bound it had "negative" energy but when you zeroed it (giving energy) it got free.
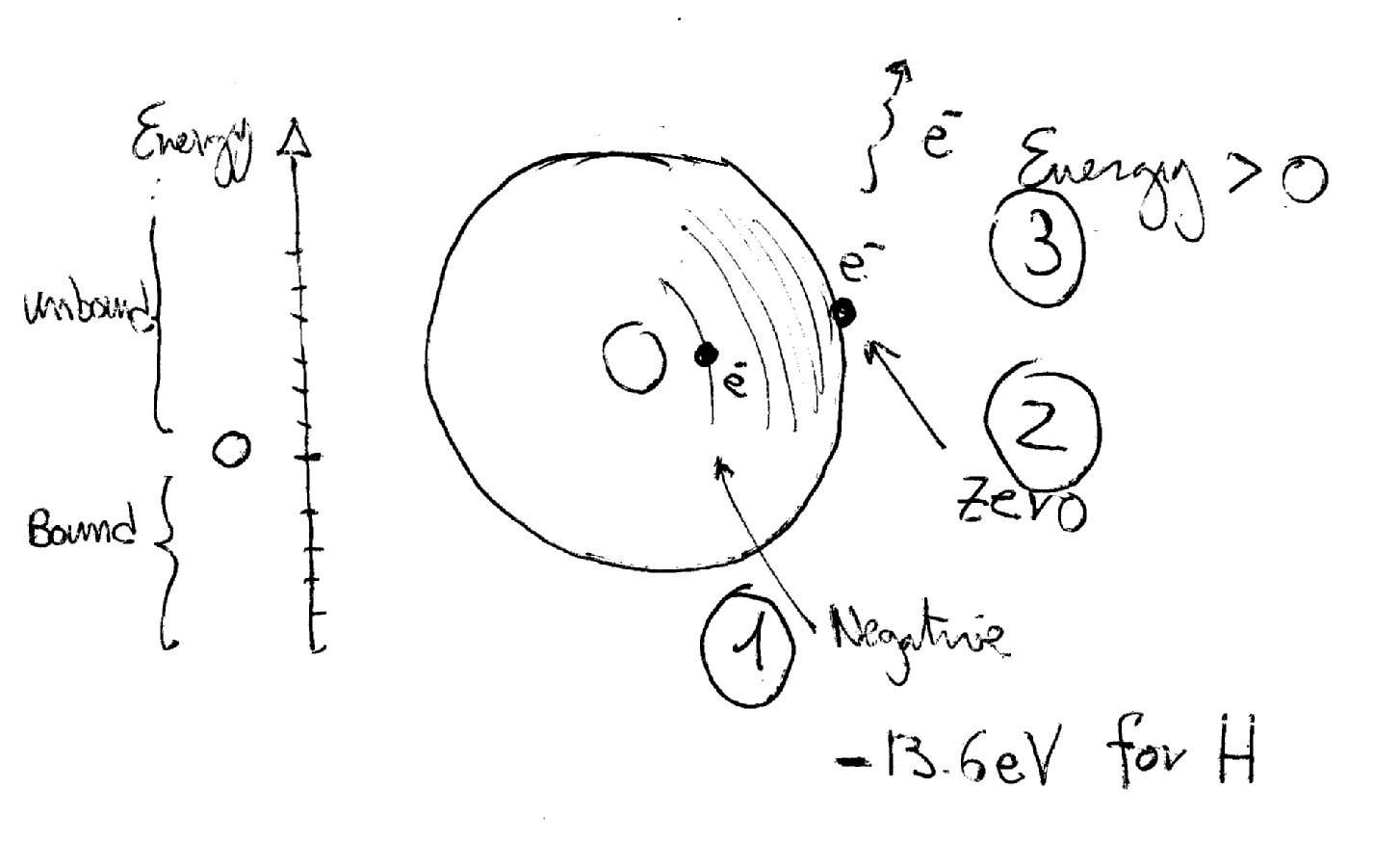
Probably it is a "simplified" explanation...but I think it works!

