A student found that the sulfate ion concentration in a solution of Al2(SO4)3 was 0.22 M. What was the concentration of Al2(SO4)3 in the solution?
1 Answer
The concentration of
So, you're dealing with an aluminium sulfate solution.
In aqueous solution, the compound will dissociate into aluminium cations and sulfate anions.
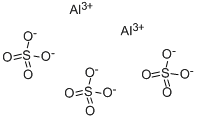
Write the equation for aluminium sulfate's dissociation (all the species are in aqueous solution)
Notice the 1 mole of aluminium sulfate produces
This means that, for a given volume of solution, the concentration of the sulfate anions will be three times larger than the concentration of aluminium sulfate.
Think of it like this - same volume, three times the number of moles in favor of the sulfate anion
Thus,

