In the combustion analysis of an unknown compound containing only carbon , hydrogen and oxygen, the grams of oxygen are found from the grams of? A) CO2 and unknown compound B) CO2,H2O and unknown compound
1 Answer
The answer is B)
The basic idea behind combustion analysis is that, when you burn a substance that contains
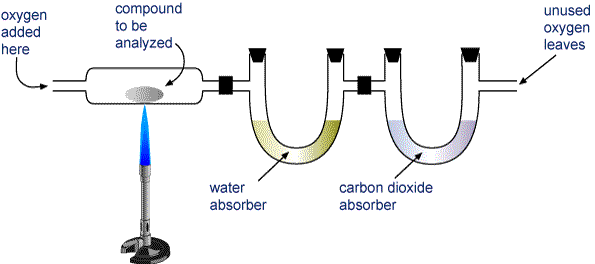
The important hing about combustion reactions for compounds that contain carbon, hydrogen, and oxygen is that they only produce carbond dioxide, water, and heat.
Notice that all the water and all the carbon dioxide are retained in the two separate tubes. If you assume that all the carbon that was a part of the original compound is now a part of the produced
Likewise, if all hydrogen is assumed to be a part of the produced
To determine how much oxygen your unknown compound contained, just subtract from the mass of the sample you burned the masses of the carbon and hydrogen you obtained.

