Question #65e17
1 Answer
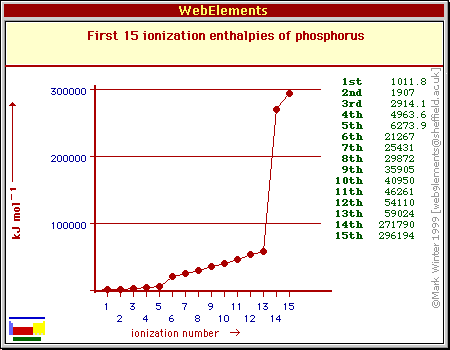
The period 3 element that has the ionization energies given is phosphorus, or
Ionization energy is defined as the amount of energy you need to remove one electron from one mole of an atom or ion in gaseous state.
Phosphorus is located in period 3, group 15 of the periodic table, and has an atomic number equal to 15, which means its electron configuration must account for 15 electrons.
Using the noble gas shorthand notation, its electron configuration is
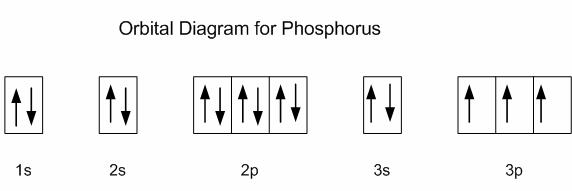
The first ionization energy of phosphorus corresponds to the removal of 1 electron from its 3p-subshell. Notice that
A noticeable increase in the energy needed to remove electrons comes when you move on to the 3s-subshell.
However, notice the very big difference between
As a consequence, electrons will become increasingly difficult to remove because of their proximity to the nucleus.
Just notice the jump in ionization energy you have when removing electrons from the first shell - roughly 271,790 kJ/mol and 296,000 kJ/mol, respectively.

