How many moles of ammonium nitrate are present in #"8.047 g"# of this substance?
1 Answer
Mar 29, 2015
That much ammonium nitrate contains
When you are asked to convert either grams to moles, or moles to grams, always think molar mass.
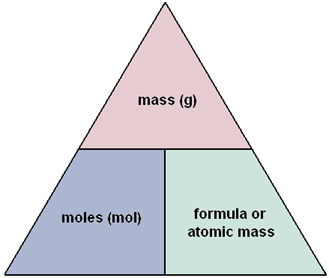
A compound's molar mass represents the weight of 1 mole of that respective compound. You can either look up the molar mass of ammonium nitrate,
Since ammonium nitrate's molar mass represents the mass of 1 mole, you can use it to determine how many moles you correspond to that many grams
In your case, ammonium nitrate's molar mass is 80.052 g/mol, which means that

